3356
Where is Pain in the Brain? Revealing Neural Correlates of Pain as Orchestration of Allostatic Domain-General Patterns via fMRI at 7T
Henning Matthias Reimann1, Jurjen Heij2, Thomas Gladytz1, and Thoralf Niendorf1,3
1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine, Berlin, Germany, Berlin, Germany, 2Spinoza Centre for Neuroimaging, Amsterdam, Netherlands, 3Experimental and Clinical Research Center, a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine, Berlin, Germany, Berlin, Germany, 2Spinoza Centre for Neuroimaging, Amsterdam, Netherlands, 3Experimental and Clinical Research Center, a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
Synopsis
Keywords: Gray Matter, fMRI, pain, allostasis
To date, fMRI has failed to distinguish an unambiguous and reliable signature that is unique to pain. Pain is accompanied by the activation of networks related to saliency processing. Employing the high sensitivity and spatial fidelity of 7T fMRI, we disentangle these overlapping representations. We apply stimuli that are perceived as painful and others that trigger saliency-related domain-general brain activity. Applying partial least square regression, we identify modality-specific characteristics in the way domain-general patterns are orchestrated relative to each other, revealing a large number of neurosignatures that are specific to the perception of pain.Intro
Functional MRI (fMRI) has been used in a number of attempts to identify objective biomarkers of pain in the brain1. This has been challenged because decoding a neural signature of pain requires distinguishing pain-specific activity from other allostatic brain functions that predict and regulate physiological processes to provide appropriate body states for a given situation1,2. Painful stimuli are accompanied by the activation of diverse networks related to salience (awareness), interoception, and autonomic control. To date, fMRI studies have failed to distinguish an unambiguous and reliable signature against this background that is unique to pain1,3. Here we use the high sensitivity, enhanced BOLD contrast and spatial fidelity of 7T fMRI to disentangle these overlapping representations via fine-grained cortical mapping. We compare painful versus non-painful control stimuli that trigger overlapping signatures of salience, interoception and other domain-general brain activities, across several sensory modes. We provide a first overview that identifies and ranks functional neurosignatures across the brain according to the degree to which they are specific to pain.Methods
Experimental design: 9 volunteers (4/5, w/m) underwent fMRI examination, each consisting of 4 runs of 12 min. Stimuli (4 stimulus types, 8 per type and run) were applied briefly (duration ~100 ms) and pseudorandomized (interstimulus spacing 18-24s): contact heat (51°C), electrical stimulation in 2 stimulus intensities: low (6mA) and mid (10 mA), and flashes of light.Data acquisition: High-resolution T2*-weighted fMRI (GE-EPI, TR/TE/FA = 2s/33.2ms/66°, FOV/matrix = 240x240mm/160x160, number of slices = 80, spatial resolution = 1.5mm isotropic, 220 volumes) was acquired on a MAGNETOM 7T scanner (Siemens Healthcare, Erlangen, Germany) with a single-channel-transmit/32-channel receive head coil (Nova Medical, Wilmington, MA, USA).
Data analysis: fMRI data were motion and distortion corrected, smoothed, registered to MNI152, and statistically analyzed using FSL FEAT. A partial least squares (PLS) model was trained and cross-validated that differentiates between the stimulus types based on individual z-stat images. The 16 images (4 per stimulus) per volunteer were normalized by their singular vector length in a singular value decomposition truncated after the 24th component. A leave one subject out cross validation was performed to ensure transferability. Further variable selection was done by excluding all voxels whose heat-pain prediction coefficients had a relative standard deviation across the 8 cross-validation models exceeding 0.4.
Results
To disentangle representations that are specific to pain from domain-general patterns, we applied brief painful heat versus non-painful salient (i.e., attention gabbing) control stimuli, including electrical stimuli of two intensities (mid and low) and flashes of light. The cortical representations we observed for all stimuli strongly overlap in domain-general regions (Fig. 1). The patterns were highly bilateral with the exception of the contralateral S1 for cutaneous stimuli which was not activated in response to visual stimuli. Visual stimuli exhibited no activation in the posterior insula, which was prominently activated in response to electrical stimuli and especially heat pain (Fig. 1B).To ensure a fair classification of pain signatures versus domain-general patterns of non-pain-specific processes, we only considered differences in voxels that were found significant for heat pain in univariate analyses (excluding driving activity in e.g., primary visual cortices). Using PLS regression, individual z-maps for distinct stimuli clustered along three major axes based on similarities in the spatial distribution of z-scores (Fig. 2A). This allowed us to predict whether a z-map was the result of painful heat versus another non-painful condition, with an accuracy of up to 97% (Fig. 2B) when only considering z-maps that exhibited average z-scores of >1.35 ("well-responders", Fig. 2A). Additionally including z-maps that exhibited average z-scores below 1.35 but clustered particularly well (Fig. 2A) further improved prediction accuracy (98%).
We used these re-classified low responders together with the well-responders to assess which voxels drive the classification of pain versus other salient stimuli. The mean covariance map that derived from this assessment depicts voxels that drive the classification of all stimuli (Fig. 3); these can be considered shared domain-general patterns. Each condition deviates from this with a particular offset. We calculated covariance coefficient maps for mean, painful and non-painful salient control conditions (Fig. 4).
Comparing covariance coefficients of painful heat with non-painful electrical stimulation reveals that although both conditions share domain-general patterns, the weighting of these patterns (i.e., how they vary from the offset) differs. This is particularly true in some areas – such as the insular (74-78) and opercular regions (42-48), where the weighting is even reversed.
By applying a brain atlas that delineates areas based on their intrinsic homotopic connectivity profile, we extracted maximum peaks of covariance coefficients across the brain and ranked them based on their impact for the classification of pain (Fig. 5).
Discussion & Conclusion
Our work presents the first fine-grained cortical cartography of representations of painful heat. By detailing these against the background of domain-general patterns elicited by non-painful salient stimuli, we identified an encoding scheme of modality-specific characteristics by differential weighting of domain-general patterns. On this basis, we were able to disentangle an extensive list of pain-specific neurosignatures from saliency-related processes, confirming some recent advances in the field1 and enriching our understanding of pain.Acknowledgements
No acknowledgement found.References
1. Lee IS, Necka EA, Atlas LY, et al. Distinguishing pain from nociception, salience, and arousal: How autonomic nervous system activity can improve neuroimaging tests of specificity. Arch Neurol. 2020;204:1053-8119.
2. Katsumi Y, Theriault JE, Quigley KS, Barrett LF. Allostasis as a core feature of hierarchical gradients in the human brain. Network Neuroscience 2022; 6 (4): 1010–1031.
3. Jabakhanji R, Vigotsky AD, Bielefeld J, et al. Limits of Decoding Mental States with fMRI. Cortex 2022; 149: 101-122.
4. Joliot M, Jobard G, Naveau M, et al. AICHA: An atlas of intrinsic connectivity of homotopic areas. J Neurosci Methods 2015; 30(254):46-59.
Figures
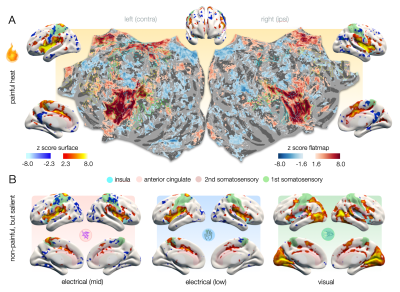
Figure 1 – A. Cortical representations of heat pain on surface renderings and hemispheric flat maps. On flat maps, delineations of homotopic areas (AICHA)4 are labeled with numbers that correspond to the areas listed in figure 5. B. Cortical representations of non-painful salient stimuli exhibit activities in domain-general pattern that largely overlap with those of pain. Posterior insula (light blue) is not activated for visual stimuli. Note the wide-spread negative effects that seem to shape cluster precision.
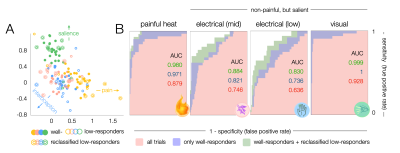
Figure 2 – Classification of pain vs non-painful salient representations. A. Clustering of z-maps based on leave-one-subject-out cross validation. Z-maps that show mean z-scores >1.35 were assigned well-responders, maps with mean z<1.35 low-responders. Low-responders that clustered well were reclassified to improve later spatial identification of voxels that drive the classification. Colors correspond to the conditions according to symbols in B. B. Receiver operating characteristic (ROC) curves show the accuracy with which a z-map can be classified to a certain condition.
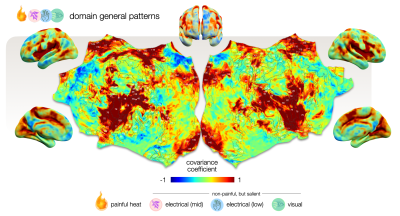
Figure 3 – Cortical map of voxels that drive the classification equally for all conditions. This mean covariance map depicts voxels that exhibit shared activity across all conditions, providing an offset from which each condition deviates. It reflects domain-general patterns that are activated in response to all kind of stimuli that yield allostatic relevance including but not specific to pain. Voxels with high (1) and low (-1) covariance coefficients impose a particularly large impact on the classification.
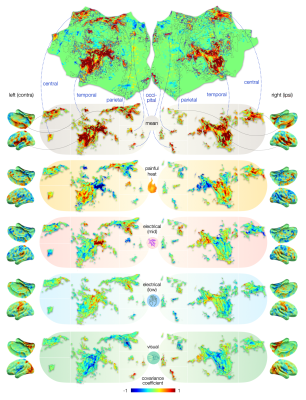
Figure 4 – Cortical maps of voxels that drive the classification of painful versus non-painful processes. Deviations from the mean covariance map drive the classification of a given condition: voxels with positive covariance coefficients deviate from with higher z-scores, negative covariances with lower z-scores for most z-maps of a given condition. Heat pain maps deviate positively in insulae (74-78) and negatively in opercular regions (42-48), which is flipped for mid electrical stimuli. Pain is likely encoded by weighting of domain-general patterns.
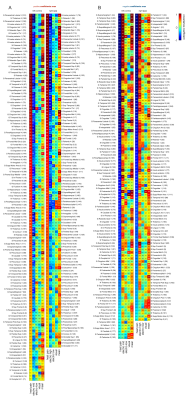
Figure 5 – Covariance peak deviations from mean that drive the classification of heat pain versus other salient conditions across the brain. Areas are ranked by maximal covariance coefficients for heat pain. Magnitude of covariance highlights the impact of voxels within a given area on the classification of pain. The higher the covariance the more likely the area is involved in the encoding of heat pain. Covariance coefficients for the mean map show whether the voxel is only activated by pain (pain-specific) or also by other stimuli that signal allostatic relevance (domain-general).
DOI: https://doi.org/10.58530/2023/3356