3354
Dynamic evolution of the predicated brain age in liver transplantation recipients: a longitudinal study1Department of Radiology, Tianjin First Central Hospital, Tianjin, China, Tianjin, China, 2MR Collaboration, Siemens Healthcare Ltd., Beijing, China, Beijing, China, 3College of Intelligence and Computing, Tianjin University, Tianjin, China, Tianjin, China
Synopsis
Keywords: Gray Matter, Machine Learning/Artificial Intelligence
To evaluate the dynamic change in overall brain health in liver transplantation (LT) recipients, we constructed a deep learning-based brain age prediction model to measure the longitudinal changes of ‘brain age’ before and one, three, and six months after surgery. The LT recipients’ brain age showed an inverted U-shaped change pattern in the early stages after transplantation. In addition, brain aging was aggravated within one month after surgery, and the patients with a history of overt hepatic encephalopathy were particularly affected. Therefore, the age prediction model can be used to monitor the post-surgical brain function recovery trajectory in LT recipients.
Introduction
Hepatic encephalopathy (HE) is a severe complication of cirrhosis with the only curative treatment being liver transplantation (LT), which was divided into minimal and overt HE (OHE)1. Neuroimaging studies have provided evidence for postoperative brain function and network plasticity in support of restorative brain function2. However, there is no reliable biomarker to assess the effect of LT on overall brain health, its recovery trajectory, and the role of preoperative OHE history. Therefore, the purpose of this study was to evaluate the dynamic evolution process of overall brain health in LT recipients by employing a deep learning-based neuroanatomic biomarker to measure longitudinal changes of ‘brain age’ before as well as one, three, and six months after surgery.Methods
We constructed a brain age prediction model based on 3D convolutional neural network (3D-CNN) through processing three-dimensional (3D) T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) images of 3609 healthy individuals from publicly accessible datasets (Methods 1). Then we further applied this model to a local dataset of 60 patients with cirrhosis, who underwent LT, and 134 healthy controls. Then, patients were divided into the OHE subgroup(n=27) and no-OHE subgroup(n=33) according to OHE history (Table 1).For the open-access dataset, high-resolution 3D T1-weighted MPRAGE images covering the whole brain were acquired on a 1.5T or 3T MRI scanner at eight centers. For the local dataset, 3D T1-weighted MPRAGE images were acquired on 3T MAGNETOM Trio a Tim System (Siemens Healthcare, Erlangen, Germany) equipped with a standard head coil (repetition time = 1900 ms, echo time = 3 ms, inversion time = 900 ms, flip angle = 9°, number of slices = 176, voxel size = 1.0 x 1.0 x 1.0 mm, and matrix size = 256 x 256).
The predicted age difference (PAD) was measured to quantitatively estimate brain age changes before and after LT by using paired t-tests, and we used a two-sample t-test to compare the difference between the OHE and non-OHE subgroups. The network occlusion sensitivity analysis (NOSA) method was used to determine the contribution of each brain network in brain age prediction.
Results
As shown in Fig.1a, the PAD of patients with cirrhosis increased markedly at baseline (+5.74 years) and continued to increase one month after LT (+9.18 years). Subsequently, the brain age began to decrease gradually but it was still higher than the chronological age (+6.46 years, 3 months post-LT; +3.60 years, 6 months post-LT). Furthermore, when we focused on the eighteen recipients who completed all baseline and three follow-up scans, the dynamic change pattern in PAD values were still similar (Fig.1b). The PAD values of the OHE subgroup were higher than those of the non-OHE subgroup, and the discrepancy was more obvious at one month post-LT (Fig.1c). Advanced cognition-related networks were more important in predicting the brain age of patients with cirrhosis at baseline, while the contribution of primary sensory networks increased temporarily within six months post LT (Fig.2).Discussion
Our results showed that at baseline (before LT), brain age in patients with cirrhosis was 5.74 years older than their chronological age, consistent with previous structural brain imaging studies. Several cross-sectional studies have found decreased global and regional brain volumes in patients with cirrhosis3-5. The brain morphology of LT recipients showed further accelerated aging over the one-month follow-up period after the operation, which may be explained by perioperative and postoperative factors, such as immunosuppressive drugs. The neurological side effects of calcineurin inhibitor, the most widely used immunosuppressive agent, have been observed for years, especially within the first few weeks after transplantation6,7. Moreover, the accelerated aging of the brain in LT recipients peaked at one month after LT before beginning to decrease progressively. The dynamic alterations in predicted brain age indicated that structural brain health was gradually restored. In addition, the PAD values of the OHE patients were significantly higher than those of the no-OHE patients in the first follow-up examination (one month after LT), indicating that OHE patients have poorer brain reserve and are more vulnerable to perioperative deterioration8, especially in the early phase after LT. Using the NOSA method, we found that the importance of primary sensory networks (VN and SMN) decreased in patients with cirrhosis but temporarily increased during the follow-up after transplantation. As lower gray matter density contributed to greater predicated age9, the temporarily increased importance in these networks might indicate reduced gray matter density caused by transient brain edema during the perioperative period. Therefore, we speculate that these areas might be the brain regions most affected by surgical and postoperative factors. Regarding the decreased importance of high-level cognition-related networks (FPN and VN), this appeared be a secondary change caused by the increased importance of primary sensory networks.Conclusion
The brain age of LT recipients showed an inverted U-shaped dynamic change pattern in the early stage after transplantation. The patients’ brain aging was aggravated within one month after surgery, and the subset of patients with a history of OHE was particularly affected. The age prediction model can be used to monitor the recovery trajectory of brain health in LT recipients after transplantation.Acknowledgements
No acknowledgement found.References
[1] Hopp AE, Dirks M, Petrusch C, et al. Hepatic Encephalopathy Is Reversible in the Long Term After Liver Transplantation. Liver Transpl. 2019. 25(11): 1661-1672.
[2] Lin WC, Hsu TW, Chen CL, et al. Reestablishing brain networks in patients without overt hepatic encephalopathy after liver transplantation. J Cereb Blood Flow Metab. 2014. 34(12): 1877-86.
[3] Lin W, Chen X, Gao YQ, Yang ZT, Yang W, Chen HJ. Hippocampal atrophy and functional connectivity disruption in cirrhotic patients with minimal hepatic encephalopathy. Metab Brain Dis. 2019. 34(6): 1519-1529.
[4] Liu K, Chen G, Ren SY, et al. Regional gray matter abnormality in hepatic myelopathy patients after transjugular intrahepatic portosystemic shunt: a voxel-based morphometry study. Neural Regen Res. 2019. 14(5): 850-857.
[5] Wang M, Cui J, Liu Y, et al. Structural and functional abnormalities of vision-related brain regions in cirrhotic patients: a MRI study. Neuroradiology. 2019. 61(6): 695-702.
[6] Rompianesi G, Montalti R, Cautero N, et al. Neurological complications after liver transplantation as a consequence of immunosuppression: univariate and multivariate analysis of risk factors. Transpl Int. 2015. 28(7): 864-9.
[7] Bernhardt M, Pflugrad H, Goldbecker A, et al. Central nervous system complications after liver transplantation: common but mostly transient phenomena. Liver Transpl. 2015. 21(2): 224-32.
[8] Ahluwalia V, Wade JB, Moeller FG, et al. The etiology of cirrhosis is a strong determinant of brain reserve: A multimodal magnetic resonance imaging study. Liver Transpl. 2015. 21(9): 1123-32.
[9] Zhang G, Cheng Y, Liu B. Abnormalities of voxel-based whole-brain functional connectivity patterns predict the progression of hepatic encephalopathy. Brain Imaging Behav. 2017. 11(3): 784-796.
Figures
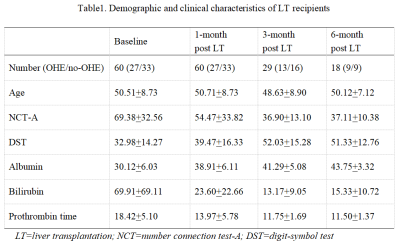
Table1. Demographic and clinical characteristics of LT recipients
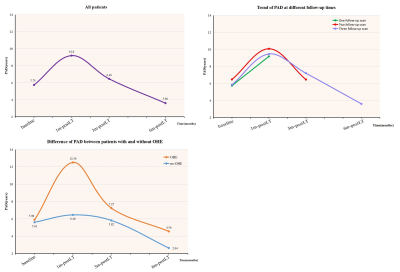
Fig.1. Inverted U-course of longitudinal changes in brain age from pre- to post-LT stages
a) For all patients, the PAD values showed an inverted U-shaped dynamic change pattern from baseline to one, three, and six months post-LT. b) For the eighteen patients who completed all baseline and follow-up scans, the PAD values showed a similar dynamic change pattern. c) The PAD values of the OHE patients changed more significantly than those of the no-OHE patients; there was a significant difference in PAD between the two groups one month post-LT.
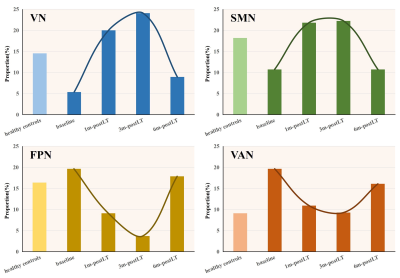
Fig. 2. Dynamic migration of brain networks
a) and b) The proportion of primary sensory networks (VN and SMN) was lower in LT recipients than in HCs at baseline. After transplantation, their proportion increased temporarily and returned to baseline six months after LT. c) and d) The proportion of high-level cognition-related networks (FPN and VAN) was higher in LT recipients than in HCs at baseline. After transplantation, their proportion decreased temporarily and returned to baseline six months after LT.