3353
Non-exponential transverse relaxation in the brain’s basal ganglia
Rita Oliveira1, Quentin Raynaud1, Valerij Kiselev2, Ileana Jelescu3, and Antoine Lutti1
1Laboratory for Research in Neuroimaging, Department of Clinical Neuroscience, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 2Division of Medical Physics, Department of Radiology, University Medical Center Freiburg, Freiburg, Germany, 3Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne, Lausanne, Switzerland
1Laboratory for Research in Neuroimaging, Department of Clinical Neuroscience, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 2Division of Medical Physics, Department of Radiology, University Medical Center Freiburg, Freiburg, Germany, 3Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne, Lausanne, Switzerland
Synopsis
Keywords: Gray Matter, Relaxometry
Non-exponential transverse relaxation in the brain’s basal ganglia has been investigated in theoretical studies but little evidence from in-vivo MRI data exists in support of this behaviour. Here, we provide experimental observation of non-exponential transverse relaxation in-vivo MRI data in the basal ganglia at 3T. The strongest deviations from exponential behaviour take place in the iron-rich pallidum and substantia nigra. Our results suggest that water diffusion through the inhomogeneous magnetic field induced by paramagnetic iron deposits may be at the source of non-exponential transverse relaxation in the basal ganglia.Introduction
Theoretical studies of the effect of magnetic material within brain tissue (e.g. iron-loaded cells) anticipate a transition from a Gaussian transverse relaxation at short echo times to an exponential decay at long echo times1–5. However, little experimental evidence exists in support of non-exponential transverse relaxation in in-vivo data of the basal ganglia, and the exponential decay rate R2* remains the primary measure to monitor microscopic brain changes in these iron-rich structures6,7.Two mechanisms exist that describe the biophysics of non-exponential relaxation in iron-rich grey matter. In the static dephasing regime (SDR), water diffusion is negligible and transverse relaxation is driven by the volume of dephased spins around the magnetic perturbers, which increases with the echo time1,2. In the diffusion narrowing regime (DNR), transverse relaxation is driven by water diffusion between magnetic perturbers2–5. The relative contribution of these two regimes to transverse relaxation was examined in an ex-vivo study of the substantia nigra6.
Here, we provide experimental evidence of non-exponential MRI transverse relaxation in basal ganglia data acquired in-vivo at 3T. We provide estimates of the Gaussian and exponential decay rates at short and long echo times and investigate which of the SDR or DNR may be the cause of this behaviour.
Theory
The signal decay can be expressed using the following Padé approximation of the crossover from Gaussian to exponential transverse relaxation:$$S=exp\left [-\frac{<\Omega^{2}>{T_{E}}^{2}}{2\left (1+\frac{<\Omega^{2}>}{2R_{2}^{*}}T_{E} \right )} \right ] \ \ \ \ \ \ \ \ [1]$$
where $$$<\Omega^{2}>$$$ is the mean square frequency deviation due to the field inhomogeneities induced by the magnetic material and $$$T_{E}$$$ is the echo time of the MRI data. $$$S \sim e^{-\frac{1}{2}<\Omega^{2}>T_{E}^{2}}$$$ at short echo times ($$$T_{E}<<\frac{2R_{2}^{*}}{<\Omega^{2}>}$$$) and $$$S \sim e^{-R_{2}^{*}T_{E}} $$$ at long echo times ($$$T_{E}>>\frac{2R_{2}^{*}}{<\Omega^{2}>}$$$) .
Within the basal ganglia, the magnetic material is assumed to consist of randomly distributed iron-rich spherical cells that occupy a fraction $$$\zeta$$$ of the voxel volume and have a characteristic frequency deviation $$$\delta\omega$$$. The following equations link the MRI signal and the properties of the magnetic material: $$$<\Omega^{2}>=0.8\zeta\delta\omega^{2}$$$; $$$R_{2}^{*}=A\zeta\delta\omega$$$ in the SDR 1 and $$$R_{2}^{*}=B\zeta\delta\omega^{2}\tau$$$ in the DNR, where $$$\tau=\frac{R^{2}}{6D}$$$ is the time scale for water molecules to diffuse away from magnetic perturbers, $$$D$$$ is the water diffusion coefficient3–5. For spherical perturbers $$$A=1.209$$$ and $$$B=1.920$$$1,3–5. The dimensionless parameter $$$\alpha=\sqrt{<\Omega^{2}>}\tau$$$ represents the dephasing due to the field inhomogeneities during $$$\tau$$$. $$$\alpha<<1$$$ ensures the validity of the DNR regime2.
Methods
The study was approved by the local ethics committee. 3D FLASH data was collected on 5 human subjects (2 females, mean 33 y.o.) with a 3T Prisma Siemens MRI scanner. 16 gradient-echo images were acquired with a bipolar readout ( $$$T_{E}$$$=1.25 to 19.25 ms, 1.2 ms spacing). Image resolution was 1.2mm isotropic. To minimize the spurious effect of cardiac pulsation, data acquisition was suspended during the systole of the cardiac cycle8.The data was fitted with Eq. [1] to obtain estimates of $$$<\Omega^{2}>$$$ and $$$R_{2}^{*}$$$. From the values of $$$R_{2}^{*}$$$ and the expression of the decay rate in the SDR, we computed estimates of bulk magnetic susceptibility $$$\zeta\Delta \chi =\zeta\delta\omega*\frac{3}{\gamma B_{0}}$$$. From the values of $$$<\Omega^{2}>$$$ and $$$R_{2}^{*}$$$, we estimated $$$\delta\omega$$$ and $$$\zeta$$$ in the SDR and $$$\zeta\delta\omega^{2}$$$ and $$$\tau$$$ in the DNR. From the values of $$$<\Omega^{2}>$$$ and $$$\tau$$$, we estimated the parameter $$$\alpha$$$ in the DNR.
Results
The log-signal decays in the basal ganglia (Fig. 1) show a transition between a quadratic dependence at short times and a linear dependence at long times, characteristic of the effect of paramagnetic inclusions2. The $$$R_{2}^{*}$$$ and $$$<\Omega^{2}>$$$ are highest in the iron-rich substantia nigra and pallidum (Fig. 2 and Table 1). The estimates of $$$\zeta\Delta \chi $$$, shown in Fig. 3 for the pallidum, are in good agreement with the literature (e.g. 0.13 ppm9).Separate estimation of $$$\delta\omega$$$ and $$$\zeta$$$ under the assumption of the SDR leads to an overestimation of $$$\zeta$$$ (~0.05) compared to literature values9 (Fig. 4a). Under the assumption of the DNR (Fig. 4b), the value of the parameter $$$\tau$$$ is ~1 ms and the value of $$$\alpha$$$ (Fig. 4b ~0.1-0.2) is consistent with the assumptions of the DNR ($$$\alpha << 1$$$).
Discussion
In the basal ganglia, transverse relaxation shows a transition between Gaussian and exponential behaviours consistent with the effects of the paramagnetic iron deposits present within the tissue. The strongest deviations from exponential behaviour take place in the iron-rich substantia nigra and pallidum regions. To investigate the microscopic mechanisms at the source of this behaviour, the parametric fitting estimates of the signal decay were linked to the properties of iron deposits under the assumption of the SDR and DNR. Our results suggest that water diffusion through the inhomogeneous magnetic field induced by paramagnetic iron deposits may be the mechanism underlying our findings, in-line with recent literature9. However, the transition times between the Gaussian and exponential behaviours lie near the shortest echo times of our data, which may prevent the accurate characterization of the Gaussian curvature with the parametric fitting approach used here9. Alternative fitting procedures should therefore be considered.Conclusions
We provide experimental evidence of non-exponential decay in in-vivo gradient-echo MRI data of the basal ganglia at 3T. This behaviour may result from the presence of iron deposits distributed within the tissue.Acknowledgements
No acknowledgement found.References
1. Yablonskiy, D. A. & Haacke, E. M. Theory of NMR signal behavior in magnetically inhomogeneous tissues: The static dephasing regime. Magn. Reson. Med. 32, 749–763 (1994).2. Kiselev, V. G. & Novikov, D. S. Transverse NMR relaxation in biological tissues. Neuroimage 182, 149–168 (2018).
3. Jensen, J. H. & Chandra, R. NMR relaxation in tissues with weak magnetic inhomogeneities. Magn. Reson. Med. 44, 144–156 (2000).
4. Sukstanskii, A. L. & Yablonskiy, D. A. Gaussian approximation in the theory of MR signal formation in the presence of structure-specific magnetic field inhomogeneities. J. Magn. Reson. 163, 236–247 (2003).
5. Kiselev, V. G. & Novikov, D. S. Transverse NMR Relaxation as a Probe of Mesoscopic Structure. Phys. Rev. Lett. 89, 2–5 (2002).
6. Brammerloh, M. et al. Measuring the iron content of dopaminergic neurons in substantia nigra with MRI relaxometry. Neuroimage 239, 118255 (2021).
7. Ye, F. Q., Martin, W. R. W. & Allen, P. S. Estimation of the iron concentration in excised gray matter by means of proton relaxation measurements. Magn. Reson. Med. 35, 285–289 (1996).
8. Raynaud, Q., Yerly, J., Heeswijk, R. B. van & Lutti, A. Characterization of cardiac noise in brain quantitative relaxometry MRI data. Proc. Intl. Soc. Mag. Reson. Med. 3908 (2022).
9. Yablonskiy, D. A., Wen, J., Kothapalli, S. V. V. N. & Sukstanskii, A. L. In vivo evaluation of heme and non-heme iron content and neuronal density in human basal ganglia. Neuroimage 235, 118012 (2021).
Figures
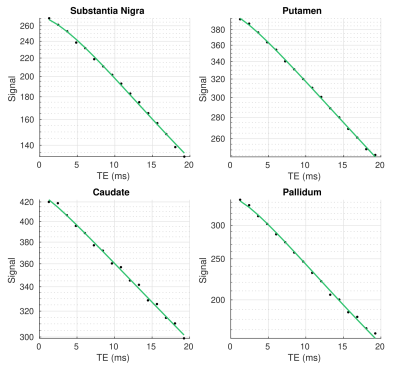
Fig. 1. Example transverse relaxation decay
data (dots) in the basal ganglia of one subject. The green lines show the fit
of the data with the Padé approximation (Eq. [1]).
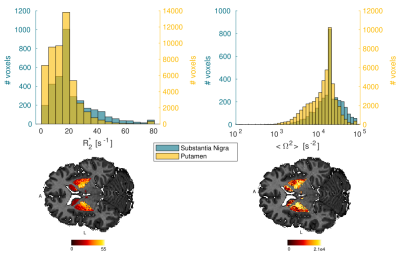
Fig. 2. Distribution of the R2* and <Ω2> estimates for the substantia nigra and putamen across participants. Maps of R2* and <Ω2> within the basal ganglia for one subject.
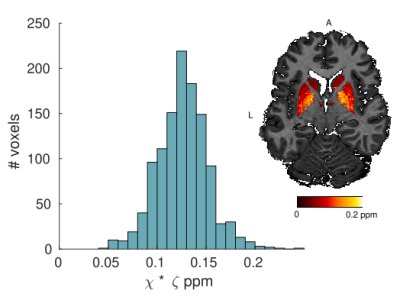
Fig. 3. Distribution of the bulk magnetic
susceptibility estimates across participants in the pallidum tissue (ζΔχ)
obtained from the parametric fits in the SDR (SI units). These estimates show a
good agreement with literature values (0.13 ppm9). Map of the bulk magnetic susceptibility within the basal ganglia for one subject.
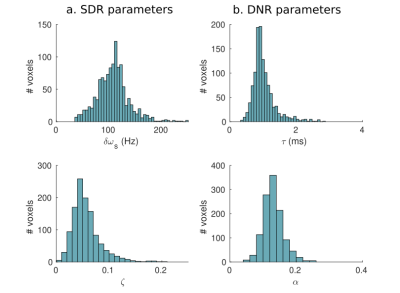
Fig. 4. Distribution of estimated magnetic perturber properties in the pallidum across participants. In the SDR (a): δω and ζ; and in the DNR (b): τ and α.
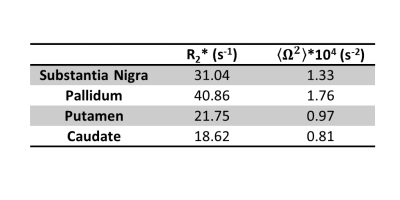
Table 1. Mean value of the parameters R2* and <Ω2> across
participants in the basal ganglia.
DOI: https://doi.org/10.58530/2023/3353