3351
Anesthetic modulation of water diffusion: Insights from a diffusion tensor imaging study1Department of Biomedical Imaging and Radiological Science, China Medical University, Taichung, Taiwan, 2Institute of Biomedical Engineering and Nanomedicine, National Health Research Institutes, Miaoli, Taiwan
Synopsis
Keywords: Gray Matter, Diffusion Tensor Imaging
A critical step in animal diffusion tensor imaging (DTI) studies is the use of anesthetics. Understanding the influence of specific anesthesia regimes on DTI-derived parameters is imperative when comparing results between animal studies using different anesthetics. Here, the quantification of fractional anisotropy (FA) and mean diffusivity (MD) under different alpha-chloralose and isoflurane is discussed. The estimated MD under isoflurane anesthesia is higher than that under alpha-chloralose anesthesia. FA quantitation was also influenced by anesthesia regimens to varying extents, depending on the brain regions and b-values. In summary, both scanning parameters and the anesthesia regimens significantly impacted quantifications of DTI indices.Introduction
Preclinical animal models are essential for translational neuroscience studies. Concurrent with this concept, the diffusion tensor imaging (DTI) technique has been widely applied to a number of animal studies to characterize brain function [1]. A critical step in designing these animal studies is the selection of anesthetics. Although all anesthetics can inhibit neurotransmitter release to exert hypnotic actions and block sensation, different anesthetics work through varying mechanisms [2], resulting in heterogeneous influence on brain physiological status, including the metabolic profile [3] and cerebral hemodynamics [4]. As the water diffusion process in vivo is a complex mechanism and is affected by numerous determinants, it is reasonable to hypothesize that changes in brain physiological status due to anesthesia may also impact DTI index quantification. Although conceptually simple, this question has not been systematically explored. In this study, we aimed to provide a comprehensive assessment of fractional anisotropy (FA) and mean diffusivity (MD) in rats anesthetized with one of two frequently used anesthetic drugs, alpha-chloralose [5] and isoflurane [1]. In particular, as DTI index quantifications in investigating tissue structural alterations were related to the diffusion-weighted factor, that is, b-value [6], we delineated the anesthetic effect using a range of b-values to account for whether the quantifications of FA and MD under different anesthetic regimes are b-value dependent.Methods
Animal preparations: A total of 13 female Sprague-Dawley (SD) rats (5 months old, 300–320g) were used in this study. The rats were divided into two groups. In the first group (n=6), each rat was anesthetized with alpha-chloralose. In the second group (n=7), each rat was anesthetized with 1.5 % isoflurane. MRI experiments: All MRI experiments were conducted using a 7T animal MRI scanner (Bruker ClinScan 70/30, Germany) with a gradient strength of 630 mT/m. DTI was performed using a single-shot echo planar imaging (EPI) sequence with the following parameters: repetition time/echo time = 6000 ms/32 ms, flip angle = 90°, field of view = 35 × 35 mm2, matrix size = 128 × 128, nine axial slices, thickness = 1.5 mm, 30 gradient directions, 4 b values of 0, 500, 1000, and 1500 s/mm2, and three averages. Data analysis: The raw DTI images were first realigned to the non-diffusion-weighted b0 images. Then, the DTI-derived MD and FA maps were calculated from the data with two b-values, namely 0 versus 500, 1000, or 1500 s/mm2, using DSI Studio [7]. Multi-slice regions-of-interest (ROIs) were manually defined in the EPI scans of each rat. These included corpus callosum, bilateral striatum, and bilateral cortex. The resulting ROI masks were then applied to the FA and MD maps to calculate regional values by averaging the values from all voxels in the ROI mask.Results
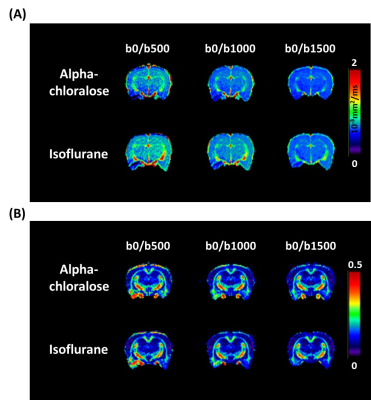
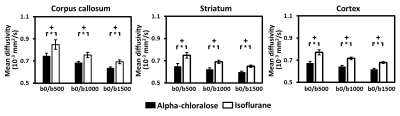
The effects of b-values on MD and FA in representative rats using different anesthesia regimens are showed in Fig. 1a and 1b, respectively. Visual inspection suggested that anesthesia-specific differences in MD were homogeneous across brain regions, regardless of the b-values.Figure 2 shows the results of ROI analysis for further investigation of the estimated MD computed using different b-values under both anesthetics. Anesthesia-specific differences in MD were apparent across different brain regions. For rats anesthetized with isoflurane, the MD was significantly higher when compared with that when animals were anesthetized with alpha-chloralose, regardless of b-values (all P < 0.001).
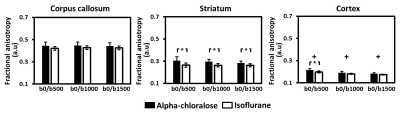
The group mean values of FA computed using different b-values under both anesthetics are illustrated in Figure 3. For FA, the anesthetic effect was regions and b-value-dependent. In the striatum, FA under isoflurane anesthesia was significantly smaller than that under alpha-chloralose anesthesia within the b-value range studied (all P < 0.05). In the cortex, the anesthesia-specific differences in FA were b-value dependent, as shown by the smaller FA under isoflurane anesthesia than that under alpha-chloralose anesthesia for the smallest b-value of 500 s/mm2 (P < 0.05). An anesthesia-related effect was not detected in the corpus callosum within the b-value range studied.
Discussion and Conclusion
The pharmacological effects of isoflurane have recently received attention, as isoflurane produces diverse effects on brain physiological conditions. One of these effects is that the potential compromise of the cell membrane under anesthesia, leading to enhanced permeability [8]. Moreover, isoflurane impairs energy metabolism, and several studies have demonstrated that isoflurane reduces baseline neural activity to a larger degree than that associated with alpha-chloralose [3]. The existing literature suggests that the shrinking of cellular elements is a reaction associated with decreased neural activity [9]. The additive effect of enhanced cell membrane permeability and shrinking of cellular elements results in augmented water diffusion, making water diffusivities (represented by MD in this study) exhibit an apparent progressive increase when rats are anesthetized with isoflurane. A reduced FA may imply water activities leaning toward less restricted isotropic diffusion [10] under the isoflurane protocol, consistent with the concept that isoflurane is characterized by enhanced cell membrane permeability [11] and shrinking cellular elements. In summary, our results stress the important notion that not only scanning parameters but the anesthesia regimens play significant roles in DTI-derived index quantification. Caution should be exercised when comparing results between animal studies using different anesthetics.Acknowledgements
No acknowledgement found.References
[1] M. Huang, L. Gao, L. Yang, F. Lin, H. Lei, Abnormalities in the brain of streptozotocin-induced type 1 diabetic rats revealed by diffusion tensor imaging, NeuroImage. Clinical 1(1) (2012) 57-65.
[2] K. Solt, S.A. Forman, Correlating the clinical actions and molecular mechanisms of general anesthetics, Current opinion in anaesthesiology 20(4) (2007) 300-6.
[3] S.L. Peng, H. Chiu, C.Y. Wu, C.W. Huang, Y.H. Chung, C.T. Shih, W.C. Shen, The effect of caffeine on cerebral metabolism during alpha-chloralose anesthesia differs from isoflurane anesthesia in the rat brain, Psychopharmacology 236(6) (2019) 1749-1757.
[4] L.P. Munting, M.P.P. Derieppe, E. Suidgeest, B. Denis de Senneville, J.A. Wells, L. van der Weerd, Influence of different isoflurane anesthesia protocols on murine cerebral hemodynamics measured with pseudo-continuous arterial spin labeling, NMR in biomedicine 32(8) (2019) e4105.
[5] J.A.A. Autio, J. Kershaw, S. Shibata, T. Obata, I. Kanno, I. Aoki, High b-value diffusion-weighted fMRI in a rat forepaw electrostimulation model at 7 T, NeuroImage 57(1) (2011) 140-148.
[6] E.S. Hui, M.M. Cheung, K.C. Chan, E.X. Wu, B-value dependence of DTI quantitation and sensitivity in detecting neural tissue changes, NeuroImage 49(3) (2010) 2366-74.
[7] F.C. Yeh, T.D. Verstynen, Y. Wang, J.C. Fernandez-Miranda, W.Y. Tseng, Deterministic diffusion fiber tracking improved by quantitative anisotropy, PloS one 8(11) (2013) e80713.
[8] J. Valette, M. Guillermier, L. Besret, P. Hantraye, G. Bloch, V. Lebon, Isoflurane strongly affects the diffusion of intracellular metabolites, as shown by 1H nuclear magnetic resonance spectroscopy of the monkey brain, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 27(3) (2007) 588-96.
[9] D. Le Bihan, S. Urayama, T. Aso, T. Hanakawa, H. Fukuyama, Direct and fast detection of neuronal activation in the human brain with diffusion MRI, Proceedings of the National Academy of Sciences of the United States of America 103(21) (2006) 8263-8.
[10] H.M. Feldman, J.D. Yeatman, E.S. Lee, L.H. Barde, S. Gaman-Bean, Diffusion tensor imaging: a review for pediatric researchers and clinicians, Journal of developmental and behavioral pediatrics : JDBP 31(4) (2010) 346-56.
[11] S. Tetrault, O. Chever, A. Sik, F. Amzica, Opening of the blood-brain barrier during isoflurane anaesthesia, The European journal of neuroscience 28(7) (2008) 1330-41.
Figures