3338
Bright Light Therapy Alters Brain Functional Connectivity in Subthreshold Depression: A Randomized Clinical Trial1Medical Imaging Center, First Affiliated Hospital of Jinan, Guangzhou, China, 2GE Healthcare, Beijing, China, Beijing, China
Synopsis
Keywords: Brain Connectivity, fMRI (resting state), Subthreshold depression
The underlying mechanisms of bright light therapy in prevention of individuals with subthreshold symptoms are unclear. This study aimed to assess the midbrain monoamine-producing nuclei treatment–related functional connectivity changes and their correlation to depressive symptom improvements in subthreshold depression. A total of 74 young adults with subthreshold depression were randomly assigned to receive 8-week BLT (N = 38) or placebo (N = 36). The dorsal raphe nucleus, ventral tegmental area, and habenula seed-based whole-brain FC were analyzed. In addition, a multivariate regression model examined whether baseline brain FC was associated with changes in scores on HDRS during BLT treatment.Background
Subthreshold depression is a risk factor for major depressive disorder, and it is known to have been associated with functional impairment, reduced quality of life, and excess mortality youths1,2. Although BLT, which is a nonpharmacological treatment3, is regarded as an effective intervention for subthreshold depression4, neural mechanisms of light therapy in the treatment of depression are unclear. Recent animal and human studies suggested depression is associated with dysfunction of the serotoninergic dorsal raphe nucleus (DRN), dopaminergic ventral tegmental area (VTA), and habenula (Hb) 5,6,7,8. However, no study has yet assessed the whole brain functional connectivity (FC) of the DRN, VTA, and Hb changes in subthreshold depression individuals who accept BLT treatment. Thus, how BLT influences the midbrain monoamine-producing nuclei and Hb function circuits, specifically in young people at risk for depression, is yet to be investigated. This study sought to examine the potential neurophysiologic mechanism underlying the effectiveness of BLT using rs-fMRI in a randomized, double-blind, placebo-controlled study.Methods
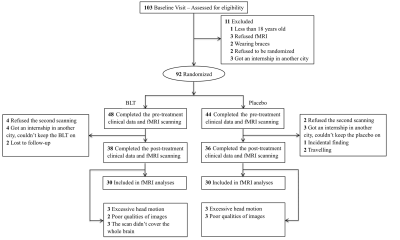
A total of 74 young adults with subthreshold depression were randomly assigned to receive 8-week BLT (N = 38) or placebo (N = 36). Depression severity was measured using the Hamilton Depression Rating Scale (HDRS) and the Center for Epidemiologic Studies depression scale (CESD). The participants underwent resting-state functional magnetic resonance imaging scans at baseline and after treatment. The DRN, VTA, and Hb seed-based whole-brain FC were analyzed. In addition, a multivariate regression model examined whether baseline brain FC was associated with changes in scores on HDRS during BLT treatment.Results
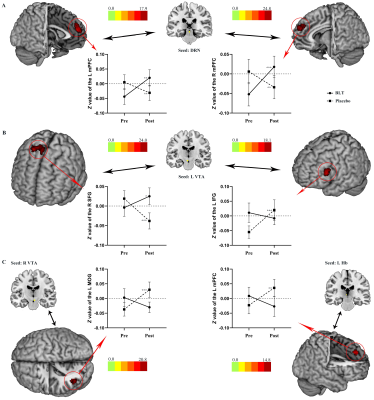
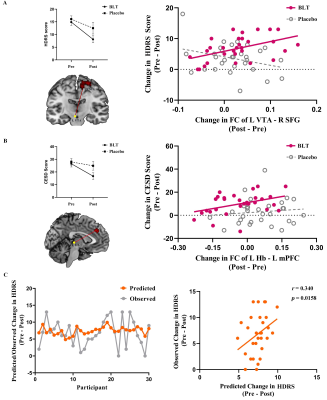
BLT group displayed significantly decreased HDRS scores and CESD scores from pre-treatment to post-treatment compared to the placebo group. BLT increased the FC between the DRN and medial prefrontal cortex (mPFC) and between the left VTA and right superior frontal gyrus (SFG). Altered VTA-SFG connectivity was associated with HDRS changes in the BLT group. Moreover, the baseline FC between DRN and mPFC could predict HDRS changes in BLT.Conclusions
These results suggested that BLT improves depressive symptoms and increases midbrain monoamine-producing nuclei (including the DRN and VTA) and frontal cortex connectivity in young adults with subthreshold depression. This phenomenon raises the possibility that pre-treatment FC of DRN-mPFC could be used as a biomarker for improved BLT treatment in depression.Acknowledgements
The study was supported by grants from the National Natural Science Foundation of China (81671670, 81971597, and 82172530); National Key Research and Development Project (2020YFC2005700); Key-Area Research and Development Program of Guangdong Province (2020B1111100001). The funding organizations play no further role in study design, data collection, analysis and interpretation and paper writing.References
1. Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J,Penninx BW.(2013): Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. The British journal of psychiatry : the journal of mental science 202:22-27.
2. Wells K, Sherbourne C, Duan N, Unützer J, Miranda J, Schoenbaum M, et al.(2005): Quality improvement for depression in primary care: do patients with subthreshold depression benefit in the long run? The American journal of psychiatry 162:1149-1157.
3. Terman M,Terman JS.(2005): Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS spectrums 10:647-663; quiz 672.
4. Pjrek E, Friedrich ME, Cambioli L, Dold M, Jäger F, Komorowski A, et al.(2020): The Efficacy of Light Therapy in the Treatment of Seasonal Affective Disorder: A Meta-Analysis of Randomized Controlled Trials. Psychotherapy and psychosomatics 89:17-24.
5. Gonzalez MM,Aston-Jones G.(2008): Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proceedings of the National Academy of Sciences of the United States of America 105:4898-4903.
6. Schlaepfer TE, Bewernick BH, Kayser S, Mädler B,Coenen VA.(2013): Rapid effects of deep brain stimulation for treatment-resistant major depression. Biological psychiatry 73:1204-1212.
7. Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al.(2013): Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493:532-536.
8. Yang Y, Wang H, Hu J,Hu H.(2018): Lateral habenula in the pathophysiology of depression. Current opinion in neurobiology 48:90-96.
Figures