3337
Intrinsic brain abnormalities in chronic rhinosinusitis and the effect of mood and cognitive function: a resting-state functional MRI study
SIMIN LIN1, MIAOMIAO NIE2, BINGSHAN WANG3, and YI HAN4
1Radiology, Xiamen Cardiovascular Hospital of Xiamen University, Xiamen, China, 2Radiology, Zhongshan Hospital of Xiamen University, Xiamen, China, 3Zhongshan Hospital of Xiamen University, Xiamen, China, 4Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, Eye Institute of Xiamen University, Xiamen, China
1Radiology, Xiamen Cardiovascular Hospital of Xiamen University, Xiamen, China, 2Radiology, Zhongshan Hospital of Xiamen University, Xiamen, China, 3Zhongshan Hospital of Xiamen University, Xiamen, China, 4Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, Eye Institute of Xiamen University, Xiamen, China
Synopsis
Keywords: Brain Connectivity, fMRI (resting state), Amplitude of low-frequency fluctuation
Chronic rhinitis (CRS) is one of the most prevalent chronic diseases that increases the risk of anxiety, depression and cognitive disorders. Here we collected rs-fMRI data to assess global brain activity and functional connectivity (FC) in CRS patients. Compared with controls, CRS patients demonstrated increased ALFF in the left orbital superior frontal cortex and decreased FC in the right precuneus cortex, with the former positively correlated with the severity of inflammation and the scores of anxiety and depression. These rs-fMRI studies provided several important hints into the potential neural mechanism related to mood and cognitive dysfunctions in CRS patients.Introduction
Chronic rhinosinusitis (CRS) is one of the most prevalent chronic diseases1. The prevalence of CRS is estimated to be more than 10% around the world 2. It is a multi-factorial disease with unclear triggers. The challenge is not only constantly coping with CRS-related symptoms, but also brain-related symptoms, such as cognitive dysfunction, depression and anxiety as recent reports3-5. Nevertheless, the pathophysiological mechanism of CRS-induced brain impairment is still largely unknown. Resting-state functional magnetic resonance imaging (rs-fMRI) is a promising technique that inspects the changes in intrinsic neural activity without task performance6. Recently, Aria et al. used rs-fMRI to study the functional connectivity of brain networks involved in cognitive processing by independent component analysis7. However, to our knowledge, there are no reports on the resting-state intrinsic brain activity in patients with CRS yet. The Amplitude of low-frequency fluctuation (ALFF) is a crucial technique to measure the low-frequency oscillation intensity and reflects the intensity of regional neural activity in a single voxel level6. Functional connectivity (FC) is an effective method to measure interregional temporal correlation and is used to reflect brain function integration8. Both approaches are involved here for the first time to explore intraregional brain activity and interregional functional connectivity. In this study, we hypothesized that 1) patients with CRS may show abnormal spontaneous neural activity and disturbed functional connectivity in resting states, and 2) the altered brain changes would be correlated with brain function disorders.Methods
A total of 26 patients with CRS and 38 age and gender-matched healthy control subjects (HCs) were included in this study. The experiment was authorized by the Ethics Committee of Zhongshan Hospital of Xiamen University. Demographic and clinical basic data were collected. The Lund-Mackay score (LMS), the visual analog scale (VAS) score, and the hospital anxiety and depression scale (HADS) scores were collected. All MRI data were obtained using a Philips Ingenia 3.0 T CX. Resting-state fMRI images were acquired by a gradient-echo-planar imaging sequence (EPI) in the axial plane. The parameters of functional images are as follows: echo time (TE) = 25 ms; repetition time (TR) = 2000 ms; thickness = 2.5mm; voxel size = 2.5 mm × 2.5 mm × 2.5mm; the number of slices =57. Structural images include high-resolution T1-weighted and T2-weighted brain images. The T1-weighted scans were acquired using a 3D magnetization-prepared rapid gradient-echo (MP-RAGE) with the following parameters: TE = 3.0 ms; TR = 6.6 ms; thickness = 1.0 mm; voxel size = 1.0 mm × 1.0 mm × 1.0 mm. The parameters of the SPIR T2-weighted images: TE = 280 ms; TR = 3000 ms; thickness = 1.0 mm; voxel size = 1.0 mm × 1.0 mm × 1.0 mm. Functional MRI data were preprocessed by DPABI software with slicing timing correction, motion correction, normalization, smoothing, linear detrending, and nuisance covariate regression, and filtering. After preprocessing, ALFF and seed-based FC analyses were calculated via REST software. Differences in ALFF and FC values between CRS and control groups were compared by independent two-sample t-test using SPM12 software. he cluster-level False Discovery Rate (FDR) method was applied for multiple comparison correction. Correlation analyses between the ALFF/FC values and clinical characteristics were further assessed in CRS patients. In addition, receiver operating characteristic (ROC) curves were conducted to access the diagnostic ability of the ALFF and FC values.Results
Twenty-eight CRS patients and 38 HCs were recruited for the current study. Details are summarized in Table 1. In comparison with the HCs, patients with CRS showed significantly increased ALFF values in the left orbital superior frontal cortex extending to the left rectus gyrus (Figure 1). FC in the right precuneus cortex was decreased when the left orbital superior frontal cortex was used as the seed point (Figure 2). In patients with CRS, ALFF values in the orbital superior frontal cortex were found to be positively correlated with the HADS-A scores, HADS-D scores and LMS, as well as a significant correlation was found between VAS and HADS-A scores (Figure 3). However, there was no significant correlation between FC alterations in the precuneus cortex and clinical index. We also performed ROC curve analysis to find potential imaging biomarkers to separate CRS patients from healthy controls. The AUC of the ALFF values of the left orbital superior frontal cortex and the FC values of the right precuneus cortex were 0.8229 (Figure 5A) and 0.7895 (Figure 5B), respectively,, indicating good accuracy.Discussion and Conclusion
This study is the first time to investigate the neural mechanisms underlying the relationship of sinonasal inflammation with anxiety and depression. The patients with CRS showed hypoactivity in the orbital superior frontal cortex (SFC), an important region in emotional regulation9, 10, and this region also shows hypoconnectivity to the precuneus cortex within the default mode network with a central role in modulating cognition11. The severity of inflammation as well as anxiety and depression scores are positively correlated with spontaneous neural activity in the orbital superior frontal cortex. These findings provide a neural basis for understanding the relationship between CRS and increased risk of mood and cognition dysfunctions, which contributes to clinical prevention and treatment.Acknowledgements
We thank all participants in this study, and this work was supported by grants from the Natural Science Foundation of Fujian Province, China (Grant No. 2015J01535).References
1. Gao WX, Ou CQ, Fang SB, et al. Occupational and environmental risk factors for chronic rhinosinusitis in China: a multicentre cross-sectional study. Respir Res. 2016;17. 2. Sedaghat AR, Kuan EC, Scadding GK. Epidemiology of Chronic Rhinosinusitis: Prevalence and Risk Factors. Journal of Allergy and Clinical Immunology-in Practice. 2022;10:1395-1403. 3. Jung HJ, Lee JY, Choi YS, et al. Chronic rhinosinusitis and progression of cognitive impairment in dementia. European Annals of Otorhinolaryngology-Head and Neck Diseases. 2021;138:147-151. 4. Kim JY, Ko I, Kim MS, et al. Relationship of Chronic Rhinosinusitis with Asthma, Myocardial Infarction, Stroke, Anxiety, and Depression. Journal of Allergy and Clinical Immunology-in Practice. 2020;8:721-+. 5. Ghadami MR. Chronic Rhinosinusitis and Increased Risk of Depression and Anxiety: The Role of Impaired Sleep Quality. Jama Otolaryngology-Head & Neck Surgery. 2019;145:689-690. 6. Guo PF, Hu SW, Jiang XL, et al. Associations of Neurocognition and Social Cognition With Brain Structure and Function in Early-Onset Schizophrenia. Frontiers in Psychiatry. 2022;13. 7. Jafari A, Xavier LD, Bernstein JD, et al. Association of Sinonasal Inflammation With Functional Brain Connectivity. Jama Otolaryngology-Head & Neck Surgery. 2021;147:534-543. 8. Lu SJ, Gao WJ, Wei ZG, et al. Intrinsic brain abnormalities in young healthy adults with childhood trauma: A resting-state functional magnetic resonance imaging study of regional homogeneity and functional connectivity. Australian and New Zealand Journal of Psychiatry. 2017;51:614-623. 9. Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116-124. 10. Subramaniam P, DiMuzio J, McGlade E, et al. Orbitofrontal Connectivity is Associated with Depression and Anxiety in Marijuana-Using Adolescents. Biol Psychiatry. 2016;79:396S-396S. 11. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564-583.Figures
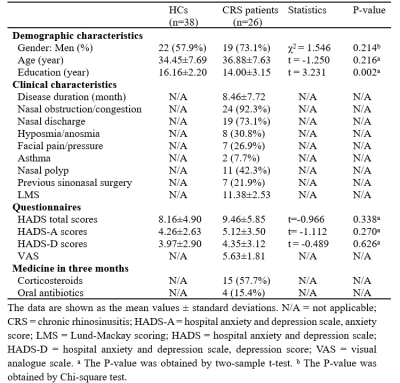
Demographic and
clinical parameters.
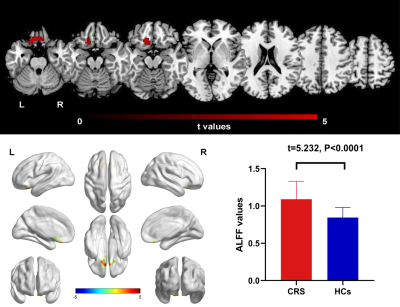
Figure 1 ALFF differences between CRS patients and HCs (P <
0.05, FDR corrected). Compared with HCs, patients with CRS showed significantly
increased ALFF in the left orbital superior frontal cortex, extending to left rectus gyrus. CRS,
chronic rhinosinusitis, HCs, healthy control
subjects, L, left, R,right.
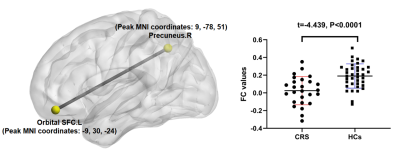
Figure
2 Seed-based
FC differences
between CRS patients and HCs (P < 0.05, FDR corrected). Compared with HCs,
patients with CRS showed significantly decreased FC between the seed region in the
left orbital superior frontal cortex and the right precuneus cortex. CRS, chronic rhinosinusitis,
HCs, healthy control subjects, SFC, superior frontal cortex, L, left, R, right.
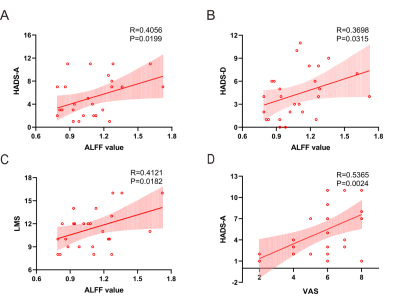
Figure 3 Correlations
between the ALFF value in the left orbital SFC and clinical assessment. (A) Correlation
between ALFF in the left orbital SFC and HADS-A scores. (B) Correlation
between ALFF in the left orbital SFC and HADSand HADS-D
scores. (C)
Correlation between ALFF in the left orbital SFC and LMS. (D) Correlation
between VAS and HADS-A scores. SFC, superior frontal cortex; HADS-A, hospital
anxiety and depression scale-anxiety; HADS-D, hospital anxiety and depression
scale-depression; LMS, Lund-Mackay scoring.
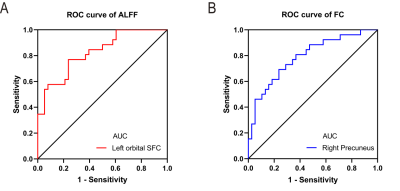
Figure 4 ROC
curve analysis for altered brain region.
(A)The AUC of the
ALFF values of the left orbital SFC was 0.8229 (P<0.001; 95% CI: 0.7211 -
0.9247); (B) the
AUC of the FCthe FC values of
the right precuneus cortex was 0.7895 (P<0.001; 95% CI: 0.6762 - 0.9028). ROC,
receiver operating characteristic; AUC, area under the curve; CI, confidence
interval; SFC, superior frontal cortex.
DOI: https://doi.org/10.58530/2023/3337