3330
Neuropathology and neurometabolites in mild cognitive impairment investigated with 11C-PiB amyloid PET and 7T MRS
Christopher William Davies-Jenkins1,2, Kathleen E Hupfeld1,2, Helge J Zöllner1,2, Gwenn S Smith3,4, and Georg Oeltzschner1,2
1The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Psychiatry and Behavioral Sciences, Johns Hopkins Medicine, Baltimore, MD, United States, 4Division of Nuclear Medicine and Molecular Imaging, The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Medicine, Baltimore, MD, United States
1The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Psychiatry and Behavioral Sciences, Johns Hopkins Medicine, Baltimore, MD, United States, 4Division of Nuclear Medicine and Molecular Imaging, The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Medicine, Baltimore, MD, United States
Synopsis
Keywords: Alzheimer's Disease, Alzheimer's Disease, MRS, PET, Amyloid, 7T, MCI
Mild cognitive impairment (MCI), a prodromal stage of Alzheimer’s disease, is increasingly studied by multi-modal beta-amyloid (Aβ) PET and MRS. Most studies have targeted the PCC and a limited number of metabolites. In this study, we analyzed Aβ PET and 7T 1H-MRS data from the ACC and PCC of MCI patients and healthy controls, using a voxel-specific Aβ metric. Metabolite-amyloid correlations were investigated using multiple regression analysis. We report a novel finding of a differential GABA-amyloid relationship for MCI patients compared with controls in the ACC and support previous reports of amyloid relationships with NAA and myo-inositol.Introduction
The pathology hallmarks of Alzheimer’s disease (AD)—and its prodromal stage mild cognitive impairment (MCI)—include the accumulation of beta-amyloid (Aβ) plaques, neurofibrillary tau protein tangles, and neurodegeneration1,2. Studies have shown that AD pathology is necessary but not sufficient to explain cognitive decline and thus other molecular mechanisms should be investigated.Multi-modal neuroimaging integrates various pathophysiological perspectives. Aβ and tau can be imaged with positron emission tomography (PET) radiotracers3,4. Magnetic resonance spectroscopy (MRS) provides complementary information about multiple MCI/AD-relevant neurobiological mechanisms, including oxidative stress (glutathione GSH); mitochondrial dysfunction (lactate Lac); neurotransmission (gamma-aminobutyric acid GABA, glutamate Glu, N-acetylaspartylglutamate NAAG); neuronal integrity (N-acetylaspartate NAA); glial activation (myo-inositol mI); membrane turnover (choline-containing compounds, tCho).
Combined PET+MRS studies are rare5–8 and focus on the posterior cingulate cortex (PCC)—a cognitive hub and prominent early Aβ accumulation site9,10—but none have investigated the anterior cingulate (ACC), despite its importance for executive function. Furthermore, combined PET+MRS studies have only been conducted at 3T, limiting the breadth of the neurometabolic profile11.
Here, we analyze Aβ-PET data together with previously reported 7T 1H-MRS data12. We expand our open-source framework (Osprey13) with volumetric PET analysis to determine MRS-voxel-specific Aβ and investigate local relationships between MRS metabolites and Aβ.
Methods
Acquisition13 MCI patients (3F, 69.6±7.7 years) and 13 healthy controls (7F, 63.6±7.8 years) were recruited. A diagnosis of control or MCI was established by the Clinical Dementia Rating scale (CDR>0.5), and a multi-domain cognitive battery.
MR data were acquired using a 7T Philips Achieva scanner (Philips Healthcare, Best, The Netherlands) with a 32-channel Tx/Rx coil, including T1-weighted anatomical imaging (3D-MPRAGE) and two STEAM acquisitions (TR = 3000 ms, TEACC = 14 ms, TEPCC = 15 ms, Nav = 96, Npts = 2048, spectral width = 3 kHz) with water-unsuppressed references (Navwater=4) in the ACC and PCC (Figure 1A).
Aβ deposition was determined with dynamic [11C]-PiB scans14 using a High-Resolution Research Tomograph (HRRT, Siemens Healthcare, Knoxville, TN). Dynamic scanning (90 minutes) began immediately after 15 mCi ± 10% radiotracer injection. Dynamic data were analyzed with the simplified reference-tissue model method to calculate the standardized uptake value ratio (SUVr), using the cerebellum as the input function15,16.
Processing and modeling
MRS data were analyzed in Osprey, using the built-in LCModel17 binary with TE-specific basis sets, and default settings for macromolecules and baseline. Metabolite levels are reported relative to total creatine (tCr). Using a newly developed feature, Osprey coregistered structural images from MRS and PET sessions with SPM12, established MRS voxel masks, and generated voxel-specific Aβ intensity distributions that were noise-filtered and modeled with a Gaussian fit (Figure 1B).
Statistical analysis
After outlier detection (CRLBs and Cook’s mean distance)12, we compared metabolite levels and Aβ between the control and MCI groups with linear models for each metabolite, and brain region and group as fixed effects. Age and sex were introduced as additional fixed effects during explorative analysis. Correlations between metabolites and Aβ were investigated separately for each brain region. For the ACC and PCC, linear models were fit to group-pooled data, with fixed effects subsequently added for age and sex. Finally, we added a fixed group*Aβ interaction to investigate whether metabolite-Aβ relationships differed between groups. All analyses were performed in R (v4.2.0)18.
Results
Figure 2 shows all spectra and an example fit. Metabolite levels showed substantial significant group effects, i.e., lower GABA and Glu and higher NAA and mI in MCI patients compared with controls (reproducing our original results12). As expected, cortical Aβ was substantially higher in MCI than in the control group.Figure 3 shows significant metabolite-Aβ correlations. ACC GABA showed significant effects for group (effect=-0.39, p=0.003), Aβ (effect=-0.19, p=0.047), and the Aβ*group interaction (effect=0.25, p=0.016), indicating different relationships between GABA and amyloid for MCI and controls. Higher NAA was consistently associated with lower Aβ in group-pooled PCC data, regardless of whether age and sex were included in the model (effect size between -0.11 and -0.09, p-value between 0.010 and 0.036). A similar result was found for tNAA (effect=-0.12, p=0.039). Finally, when controlling for [higher] mI in older age (effect=-0.01, p=0.021), mI was positively correlated with Aβ in group-pooled ACC data (effect=0.10, p=0.043).
Discussion and conclusion
This study investigates associations between local metabolite and Aβ measures. Multi-modal neuroimaging of MCI/AD is rare, and this study is the first to include the ACC and several low-concentration metabolites (e.g., GABA).We found a novel GABA-Aβ association in the ACC. One previous study7 examined the GABA-Aβ relationship, but only in the PCC, finding reduced GABA levels in MCI, but no correlation with Aβ, confirmed by our results. Connections between proteinopathy and GABAergic neurotransmission in MCI patients may mediate cognitive decline, perhaps affected by genetic risk factors7.
The negative NAA-Aβ correlation in the PCC supports two previous studies in the PCC6 and Aβ-positive regions7. Of those reporting mI, one study found a positive mI-Aβ relationship consistent with our results6, whereas another did not8. The small sample size of this study is a limitation, particularly after quality-related exclusions for low-concentration metabolites. Future work may cover other relevant brain regions (medial-temporal lobe/hippocampus) and include additional PET measurements (tau, glucose).
Acknowledgements
This work was supported by NIH grants P50AG005146, R01AG038893, R01AG041633, R01AG059390, K00AG068440, R00AG062230, P41EB015909.References
- Breijyeh Z, Karaman R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules. 2020;25(24):5789. doi:10.3390/molecules252457892.
- Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N Engl J Med. 2012;367(9):795-804. doi:10.1056/NEJMoa12027533.
- Small GW, Kepe V, Ercoli LM, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355(25):2652-2663. doi:10.1056/NEJMoa0546254.
- Pike KE, Savage G, Villemagne VL, et al. β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(11):2837-2844. doi:10.1093/brain/awm2385.
- Zeydan B, Deelchand D, Tosakulwong N, et al. Decreased Glutamate Levels in Patients with Amnestic Mild Cognitive Impairment: An sLASER Proton MR Spectroscopy and PiB-PET Study. J NEUROIMAGING. 2017;27(6):630-636. doi:10.1111/jon.124546.
- Sheikh-Bahaei N, Sajjadi SA, Manavaki R, McLean M, O’Brien JT, Gillard JH. Positron emission tomography–guided magnetic resonance spectroscopy in Alzheimer disease. Ann Neurol. 2018;83(4):771-778. doi:10.1002/ana.252027.
- Riese F, Gietl A, Zolch N, et al. Posterior cingulate gamma-aminobutyric acid and glutamate/glutamine are reduced in amnestic mild cognitive impairment and are unrelated to amyloid deposition and apolipoprotein E genotype. Neurobiol AGING. 2015;36(1):53-59. doi:10.1016/j.neurobiolaging.2014.07.0308.
- Chen Q, Abrigo J, Liu W, et al. Lower Posterior Cingulate N-acetylaspartate to Creatine Level in Early Detection of Biologically Defined Alzheimer’s Disease. Brain Sci. 2022;12(6). doi:10.3390/brainsci120607229.
- Shinno H, Inagaki T, Miyaoka T, et al. A decrease in N-acetylaspartate and an increase in myoinositol in the anterior cingulate gyrus are associated with behavioral and psychological symptoms in Alzheimer’s disease. J Neurol Sci. 2007;260(1):132-138. doi:10.1016/j.jns.2007.04.01710.
- Mihara M, Hattori N, Abe K, Sakoda S, Sawada T. Magnetic resonance spectroscopic study of Alzheimer’s disease and frontotemporal dementia/Pick complex. NeuroReport. 2006;17(4):413-416. doi:10.1097/01.wnr.0000203353.52622.0511.
- Pradhan S, Bonekamp S, Gillen JS, et al. Comparison of single voxel brain MRS AT 3T and 7T using 32-channel head coils. Magn Reson Imaging. 2015;33(8):1013-1018. doi:10.1016/j.mri.2015.06.00312.
- Oeltzschner G, Wijtenburg SA, Mikkelsen M, et al. Neurometabolites and associations with cognitive deficits in mild cognitive impairment: a magnetic resonance spectroscopy study at 7 Tesla. Neurobiol Aging. 2019;73:211-218. doi:10.1016/j.neurobiolaging.2018.09.02713.
- Oeltzschner G, Zöllner HJ, Hui SCN, et al. Osprey: Open-source processing, reconstruction & estimation of magnetic resonance spectroscopy data. J Neurosci Methods. 2020;343:108827. doi:10.1016/j.jneumeth.2020.10882714.
- Klunk WE, Debnath ML, Pettegrew JW. Development of small molecule probes for the Beta-amyloid protein of Alzheimer’s Disease. Neurobiol Aging. 1994;15(6):691-698. doi:10.1016/0197-4580(94)90050-715.
- Price JC, Klunk WE, Lopresti BJ, et al. Kinetic Modeling of Amyloid Binding in Humans using PET Imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25(11):1528-1547. doi:10.1038/sj.jcbfm.960014616.
- Zhou Y, Endres CJ, Brašić JR, Huang SC, Wong DF. Linear regression with spatial constraint to generate parametric images of ligand-receptor dynamic PET studies with a simplified reference tissue model. NeuroImage. 2003;18(4):975-989. doi:10.1016/S1053-8119(03)00017-X17.
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260-264. doi:10.1002/nbm.69818.
- Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. HttpwwwR-Proj. Published online 2016. Accessed November 2, 2022. https://cir.nii.ac.jp/crid/1574231874043578752
Figures
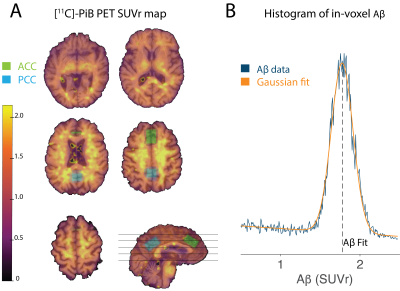
Figure 1: A) [11C]-PiB PET SUVr map for a single participant overlaid on the corresponding T1-weighted structural image. Axial slice locations are shown as black lines on a single coronal image. The two MRS voxel locations in the ACC (green) and PCC (blue) are also overlaid. B) Example of voxel-specific Aβ distribution and corresponding Gaussian fit. The Aß metric—defined as the center of this fit—is used for further analysis.
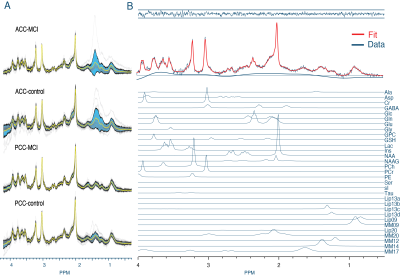
Figure 2: A) Visualization of the processed spectra of all subjects in black, with the standard deviation and mean overlaid in blue and yellow, respectively. B) Example fit of a single subject. From top to bottom: MRS fit residuals, fit in red overlaid on the data and baseline, and the individual metabolite contributions stacked below.

Figure 3: Statistically significant partial correlations of metabolites with Aβ, including fits (bold lines) and confidence intervals (shaded ribbons). A) ACC GABA was associated with Aβ with a group interaction term, suggesting different GABA-Aβ relationships between the healthy (red) and MCI (blue) groups. B) Higher ACC mI associated with higher Aβ—when including age as a fixed effect—in group-pooled data. C) Higher NAA associated with lower Aβ for group-pooled PCC data. D) Higher tNAA associated with lower Aß for group-pooled PCC data.
DOI: https://doi.org/10.58530/2023/3330