3316
Sex dimorphism in early Alzheimer’s pathology: Brain connectivity and Behavior in AppNL-F/MAPT double knock-in mice
Inès Ben Abdallah1,2, Marion Sourty1, Mary Mondino1, Laetitia Degiorgis1, Julien Lamy1, Vincent Noblet1, Marion Rame1, Cristiana Pistono2, Aminé Isik2, Marie-Dominique Marinutti2, Céline Héraud2, Hiroki Sasaguri3, Shoko Hashimoto3, Takashi Saito3, Takaomi Saido3, Chantal Mathis2, and Laura Harsan1,4
1ICube, Université de Strasbourg-CNRS, Strasbourg, France, 2LNCA, Université de Strasbourg-CNRS, Strasbourg, France, 3RIKEN Center for Brain Science, 2-1 Hirosawa, Wako-city, Saitama, Japan, 4Department of Biophysics and Nuclear Medicine, University Hospital of Strasbourg, Strasbourg, France
1ICube, Université de Strasbourg-CNRS, Strasbourg, France, 2LNCA, Université de Strasbourg-CNRS, Strasbourg, France, 3RIKEN Center for Brain Science, 2-1 Hirosawa, Wako-city, Saitama, Japan, 4Department of Biophysics and Nuclear Medicine, University Hospital of Strasbourg, Strasbourg, France
Synopsis
Keywords: Alzheimer's Disease, fMRI (resting state), Preclinical
MRI is a unique tool to study the complexity of functional and structural communication in the brain. To explore the brain architecture of a mouse model of Alzheimer's Disease and highlight sex-dimorphism in the emergence of AD-like signs, we used a recent mouse model, the APPNL-F/MAPT double knock-in (dKI). In preclinical imaging, we used resting-state graph theory approaches in a longitudinal study associated with behavioral evaluation. Functional connectivity of perirhinal, dorsal-hippocampus and midbrain nodes were implicated in early memory impairments in dKI female mice. Interestingly, perirhinal-cortex and dorsal-hippocampus are key regions for object-place associative memory and long-term object-recognition.Introduction
Alzheimer’s disease (AD) is the most common cause of dementia with more than 47M cases worldwide(1). Its early phase is characterized by a slow and progressive decline in episodic and spatial memories. 2/3 of people affected by this disease are women. Preclinical research in mouse models of AD often report age-dependant cognitive deficits but sex dimorphism in early AD is largely unexplored(2). To characterize this dimorphism in the emergence of AD-like signs, we used a recent mouse model: the AppNL-F/MAPT double knock-in (dKI)(3). The KI of these humanized AD-related genes leads to the physiological expression of amyloid precursor protein (APP) bearing two familial AD mutations and the six isoforms of normal human Tau. Hence, the model is characterized by a slow and progressive evolution of the pathology(4). We investigated sex dimorphism (i) in subtle memory deficits overtime using behavioral tests(5) and (ii) through functional and structural connectivity mapping with resting-state functional MRI (rsfMRI) and diffusion tensor imaging (DTI). Our goal was to characterize cognitive performances and their relationship with brain networks configurations at an early phase of the pathology.Methodology
Animals: The AppNL-F/MAPT dKI mice were bred on a C57BL6/J background. Experiments enrolled four groups of mice (n=17/group): dKI female and male groups and wild type (WT) female and males, imaged at 2 and 4-months each.Behavioral tests: Spontaneous object exploration tests were used to evaluate subtle impairments in 24h long-term Object Recognition (RO24h) and 5min short-term Object in Place associative memory (OiP).
MRI data acquisition: Brain imaging was performed using a 7T small bore animal scanner and a 1H four-channel phased array receive-only MRI mouse brain CryoProbe combined with a volume transmission coil 86mm diameter and the ParaVision 6.01 (Bruker, Germany). Anatomical images were acquired with a Turbo-RARE T2-weighted sequence (TE/TR=20ms/6000ms; resolution=0.07x0.07x0.35mm3; 11min), rsfMRI images with a GE-EPI sequence (TE/TR=15ms/2137ms; resolution=0.14x0.18x0.35mm3; 500 volumes; 17min), and DTI using a DTI-EPI sequence (TE/TR=44ms/6000ms; resolution=0.153; b=1000s/mm2; 40 diffusion directions; 43min). During fMRI acquisitions, mice were anesthetized by a combination of isoflurane (0.5%) and medetomidine (0.1mg.kg-1 bolus sc)(6,7,8,9). DTI and anatomical data were collected under isoflurane anesthesia (1,5%).
Data analysis: (i) Behavioral data were analyzed through memory index with a mixed-effect model. (ii) RsfMRI data were corrected for intensity bias, inter-slice-drift and motion. A band-pass filter (0.01-0.1Hz) was applied. Brain images were spatially normalized to the Allen Brain Atlas and 40 ROIs covering major brain areas were selected. From each ROIs, the average BOLD time-course was extracted and functional connectivity (FC) matrices were generated using partial correlation. The matrices were Fisher transformed to obtain the z-scores associated to the connections’ weight between pairs of ROIs and used as inputs for graph theory measures(10). Intergroup statistical comparison of connectivity matrices (2-sample t-test, p<0.01, uncorr.) was performed to identify the most different connections. Machine learning logistic regression (using a sigmoid function) was used to reveal the nodes for which the connectivity features are most discriminative between groups.
Results
Behavioral phenotyping revealed deficits in early AD-sensitive recognition memory paradigms. In both RO24h and OiP tasks, only female dKI mice were significantly impaired at 2-months. At 4-months, both dKI males and females showed a deficit (both p<0.006)(Fig.2A,B). RsfMRI showed genotype and sex related FC modifications. In females, differences between WT and dKI involved connections with the perirhinal-cortex. Indeed, perirhinal-cortex changed its connectivity with frontal pole, orbital, entorhinal, infralimbic, primary somatosensory, piriform areas and septum (Fig.3A) in females dKI. Less differences were identified in males (WT vs. dKI t-test, p<0.01) and involved connections between: midbrain-anterior cingulate cortex, midbrain-retrosplenial, amygdala-dorsal hippocampus, gustatory cortex-ventral hippocampus and midbrain-amygdala (Fig.3B). Interestingly, the retrosplenial cortex was previously identified as a dysfunctional hub in dKI males submitted to the OiP task (11). Finally, females and males dKI mice showed differences in the strength of hippocampus-midbrain, perirhinal-ventral pallidum and pallidum-visceral connections (Fig.3C). Machine learning logistic regression pointed out the connectivity of perirhinal-cortex, dorsal hippocampus and midbrain nodes (score >0.7) as most discriminative between females and males dKI; suggesting that these areas are implicated in the occurrence of early memory impairments in dKI female mice. Particularly, the perirhinal-cortex and dorsal hippocampus are key regions for object-place associative memory and long-term OR (Fig.3D).Discussion
We combined here brain FC mapping and recognition memory paradigms in the AppNL-F/MAPT dKI mouse model of AD to examine comparatively in male and female mice the early stages of the pathology. Our results indicate a female vulnerability; indeed, only dKI females showed deficits in recognition memory test at 2-months, whereas males only showed these two deficits two months later. RsfMRI data revealed differences between WT and dKI females in perirhinal-entorhinal and perirhinal-orbital connections, known to be recruited in OiP, RO24h and targets for AD pathology. Moreover, these data point out perirhinal, dorsal hippocampus and midbrain areas as potential players in sex specific memory impairments in dKI mice at 2-months; and therefore, possible vulnerable areas in females dKI. Overall, the dKI mouse model showed good promises in the characterization of the female vulnerability at early AD pathology. Further sex specific alterations in memory networks are currently being analyzed using functional (seed-based analysis, ICA(12)), structural(13) (tensor parametric maps, tractography(14), and voxel based-morphometry(15)) MRI at 4 and 12-months.Acknowledgements
No acknowledgement found.References
- World Health Organization, The epidemiology and impact of dementia, 2015
- Medeiros et al., J Alzheimers Dis, 2019 Saito et al., Nat Neurosci, 2014
- Saito et al., Nat Neurosci, 2014
- Saito et al., J Biol Chem, 2019
- Mathis, Handbook of Object Novelty Recognition, Chapter 21, 2018
- Grandjean et al., NeuroImage, 2014
- Bukhari et al., Sci Rep, 2018
- Reimann et al., Front System Neurosci, 2020
- Steiner et al., Front Neurosci, 2020
- Sporns et al., Nature Methods, 2013
- Borcuk et al., Aging Brain, 2022
- Zerbi et al., Neuroimage, 2015
- Karatas et al., Brain Structure and Function, 2021
- Tournier et al., NeuroImage, 2019
- http://www.fil.ion.ucl.ac.uk/spm/
Figures
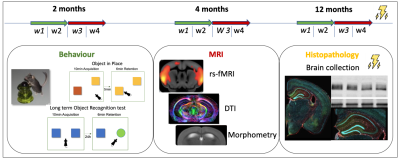
Figure 1: Experimental design. At each time point, the two first weeks (w1+w2) were dedicated to behavior experiments and the two last weeks (w3+w4) to MRI acquisitions. At 12 months old – the final time point – brains will be collected for western blotting and histopathology.
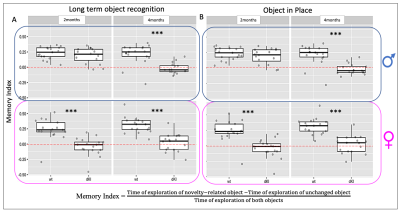
Figure 2: Behavioral phenotyping in recognition memory paradigms. A, 24h long-term Object Recognition and B, 5min short-term Object in Place associative memory. In both recognition memory tests at 2-months, dKI females showed deficits whereas males only showed these two deficits two months later. Mixed-effects model; fixed effects for sex, genotype, age and random effect for the subject. Estimation of contrasts and pvalue correction for multiple comparisons. *** pvalue < 0.006
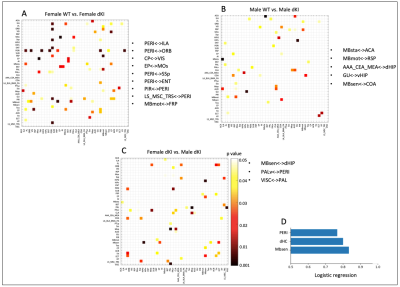
Figure 3: Functional connectivity differences between groups at 2 months. Inter-group comparison of partial correlations matrices between (A) female WT vs. female dKI, (B) male WT vs. male dKI, (C) female dKI vs. male dKI (t-test, p value<0.01). Most significantly different connections (p value<0.01) are represented in dark red and listed next to the corresponding matrix. D) Machine learning logistic regression indicates that PERI, dHC and Mbsen are the most significantly different nodes when comparing females dKI vs. males dKI. Significative scores >0.7.
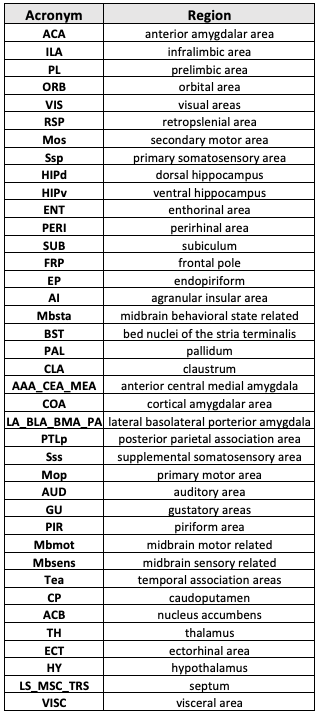
Table 1: List of 40 bilateral brain areas (nodes) evaluated.
DOI: https://doi.org/10.58530/2023/3316