3313
Pupil-fMRI correlation mapping of awake transgenic 5xFAD mice of Alzheimer’s Disease1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States, 2School of Traditional Medicine, Southern Medical University, Guangzhou, China, 3Department of Neuroscience, Boston University, Boston, MA, United States
Synopsis
Keywords: Alzheimer's Disease, Alzheimer's Disease, pupil dynamics
This study obtained visually stimulated high-resolution fMRI and real-time pupil dynamic signals in awake normal and AD mice. By analyzing the trial-specific pupillary responses to light (PRL), we identified unique temporal dynamic features in AD mice. By performing the pupil-fMRI event-related analysis, we detected specific subcortical functional nuclei in AD mice, showing strong correlations to the pupil dilation recovery features and the variability across different stimulation events. These results present novel function-behavioral linkage based on the pupillary responses to visual stimulation in AD mice, revealing an intriguing non-invasive and quantitative biomarker of the neurodegenerative progress of AD brains.Purpose
Alzheimer's disease (AD) leads to neurodegeneration of the brain, causing cognitive decline which is mainly evaluated by behavioral tests. Pupil size changes rely on environmental luminance and present brain state fluctuation corresponding to emotion, attention, and vigilance. Also, irregular pupillary responses to visual stimuli have been reported in AD patients1–4. Mapping the pupil-fMRI relationship will elucidate the function-behavioral linkage of degenerative AD brains. We developed high-resolution awake mice fMRI with real-time pupillometry5,6. The concurrent whole-brain fMRI signals can be correlated with specific pupillary response features of normal and AD mice, highlighting multiple functional nuclei across different neuromodulatory pathways. This result presents high variability of pupillary responses and its correlation to subcortical and brainstem nuclei, supporting the designated pupil dynamic changes as a non-invasive and quantitative biomarker of the degeneration process of AD brains.Materials and Methods
Data recording:The awake mouse fMRI images were obtained with a multi-slice 2D EPI (TE=6.2ms, TR=1s with two segments, NR=205, 100x100x200µm resolution) scan using a 14T ultra-wide horizontal bore scanner. The pupil recordings were obtained in a 3D-printed animal cradle with a camera and three fiber optics used as light sources (red light for video illumination, and green and blue light for visual stimulation).
Ten C57BL/6J wild-type (WT) mice and Ten 5xFAD transgenic AD mice with implanted 600MHz single-loop surface coils were used. Whole brain fMRI and real-time pupillometry were performed in well-trained awake mice (three trials per mouse per scanning section). Each trial consists of 10 visual stimulation epochs (8s on, 32s off) in a block-design paradigm. The “8s on” optical stimulation consists of two LED lights flashing (488nm 5Hz, 530nm 5.1Hz, duration 20ms). Five scans (10s) were obtained before stimulation as the baseline.
Data Analysis:
The edge of the mouse pupil was detected and tracked using Deeplabcut7. An alternative method based on ocular and global grayscales was proposed for pupil size quick detection. The pupil dynamics data is processed with z-score normalization.
For the pupil-fMRI analysis, the dilated pupil size, pupil relaxation duration, and variability of PLR were calculated as pupil dynamic features. The amplitude of the recovered pupil size dilation (A-ReDila) was used as the dilated pupil size. The pupil relaxation duration (P2-P1) is defined as the time from when the pupil is constricted to 75% of its minimum value (P1) to when the pupil is dilated to 75% of its maximum value (P2). The mean PLR of each group was used as the PLR-model. In each group, the maximum cross-correlation coefficients (MCC) between each PLR and the model were calculated. The variability of PLR can be represented by 1-MCC. The fMRI images were processed and analyzed with the software of Analysis of Functional Neuroimages (AFNI)8,9. After resampling to 100x100x100um, the EPI dataset was aligned to an anatomical template and then despiked, blurred, motion and outlier corrected, and normalized, before running amplitude modulated (AM2) linear regression using the pupil dynamic features. ANOVA was applied for group analysis.
Results
Fig 1 shows the fMRI setup with real-time pupillometry of awake mice with visual stimulation. Two traces of pupil dynamics were presented using Deeplabcut and ocular-grayscale-based methods, of which the ocular-grayscale method shows more stability for the MR in-bore pupillometry data (Fig 1C). The mean PLR shows that the recovery of light-induced pupil constriction is slower in AD mice than in WT mice, and pupil dilation in AD mice was gradually limited as the number of stimuli increased. Also, the cross-correlation analysis of the individual PLR events to the PLR-model showed lower coefficients and higher variability of the lag times in AD mice than in WT mice (Fig 2). Also, the epoch-specific analysis across different trials showed higher variability in AD mice, indicating the high irregularity of PLR (Fig 2C). Fig 3 shows the fMRI correlation maps to the visual stimuli in both AD and WT mice, highlighting strong positive BOLD signals in the superior colliculus (SC) and the lateral geniculate nucleus (LGN). The responses in the visual cortex were not robust in both cases, which could be caused by the less responsive of cortical neurons to light exposure with less luminance contrast.The AM2-based linear regression analysis enables the voxel-wise correlation analysis to unique pupil dynamic features of PLR after each visual stimulus: A-ReDila, P2-P1, 1-MCC. We performed a voxel-wise student’s t-test to compare the AM2-based correlation maps between AD and WT mice. Fig 4 shows that: i. for A-ReDila, AD mice showed significantly higher AM modulation at the parabrachial nuclei (MPB) and dorsal raphe (DR), posterior hypothalamic nucleus (PH), and basal forebrain (BF); ii. for P2-P1, AD mice showed significantly higher AM modulation at the DR only; iii, for 1-CC, we observed significantly higher correlation in the periaqueductal gray (PAG), retrosplenial cortex (RsC), and ventral tegmental Area (VTA).
Discussion
The voxel-wise pupil-fMRI correlation maps of awake mice reveal multiple function nuclei responsible for the irregular PLR in AD mice, presenting a robust function-behavioral linkage in degenerative brains.Conclusions
We have reported a novel high-resolution awake mouse fMRI methodology to bridge the function and pupil dynamic behavior of AD mice, providing solid evidence to monitor the pupillary response as a potential biomarker of the neurodegeneration of AD patients.Acknowledgements
This research was funded by NIH Brain Initiative funding (RF1NS113278, RF1NS124778, R01NS122904, R01NS120594, R21NS121642), NSF grant 2123971, and the S10 instrument grant (S10 MH124733–01) to Martino’s Center.
References
1. El Haj, M. & Moustafa, A. Alzheimer’s disease in the pupil: pupillometry as a biomarker of cognitive processing in Alzheimer’s disease. 2022;77–85.
2. Granholm, E. L. et al. Pupillary Responses as a Biomarker of Early Risk for Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2017; 56:1419–1428.
3. Kremen, W. S. et al. Pupillary dilation responses as a midlife indicator of risk for Alzheimer’s disease: association with Alzheimer’s disease polygenic risk. Neurobiol Aging. 2019;83:114–121.
4. Chougule, P. S., Najjar, R. P., Finkelstein, M. T., Kandiah, N. & Milea, D. Light-Induced Pupillary Responses in Alzheimer’s Disease. Front Neurol.2019;10.
5. Sobczak, F., Pais-Roldán, P., Takahashi, K. & Yu, X. Decoding the brain state-dependent relationship between pupil dynamics and resting state fMRI signal fluctuation. Elife. 2021;10:e68980.
6. Pais-Roldán, P. et al. Multimodal assessment of recovery from coma in a rat model of diffuse brainstem tegmentum injury. Neuroimage. 2019;189:615–630.
7. Nath, T. et al. Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat Protoc. 2019;14:2152–2176.
8. Cox, R. W. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29:162–173.
9. Cox, R. W. & Hyde, J. S. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–178.
Figures
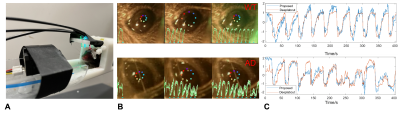
Fig 1 (A)The 3D printed animal cradle for fMRI and real-time pupillometry. (B) Two traces of pupil dynamics detected using Deeplabcut. (C) Two examples of the pupil dynamics detection results of the proposed ocular-grayscale method and Deeplabcut.
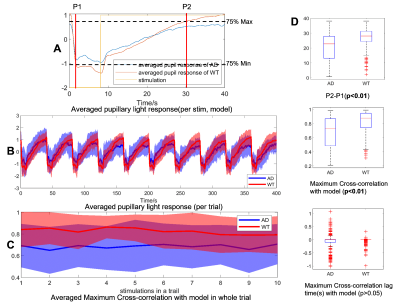
Fig 2 (A) The PLR model of AD and WT mice, and the stimulation (reversed for cross-correlation calculation). (B) The trial-averaged PLR (mean±std) of AD and WT mice. (C) The trial-averaged MCC between PLR and the model(mean±std) of AD and WT mice. (D) The box plot of P2-P1 and cross-correlation analysis results of AD and WT mice.
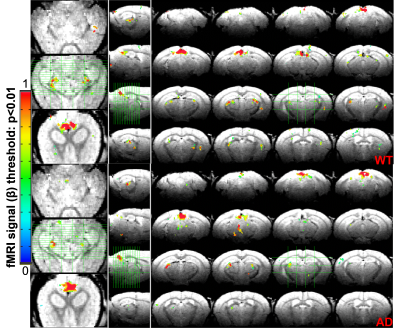
Fig 3 The averaged fMRI correlation maps to the visual stimuli in both AD and WT mice.
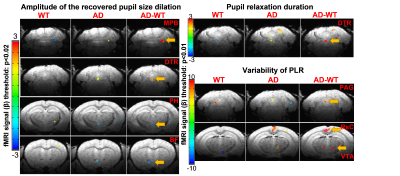
Fig 4 The averaged fMRI correlation maps to the visual stimuli with different pupil dynamic features as amplitude factors in both AD and WT mice, and the contrast of AD and WT mice.