3309
Uncertainty-based Quality Control for Subcortical Structures Segmentation in T1-weighted Brain MRI1Univ. Grenoble Alpes, Inserm, U1216, Grenoble Institut Neurosciences, GIN, Grenoble, France, 2Pixyl, Research and Development Laboratory, Grenoble, France, 3Univ. Grenoble Alpes, Inria, CNRS, Grenoble INP, LJK, Grenoble, France
Synopsis
Keywords: Machine Learning/Artificial Intelligence, Artifacts
The performance of Deep Learning (DL) models may drastically drop in the presence of characteristics in test images not present in the training set. Then, the automatic detection of these Out-Of-Distribution (OOD) inputs is important to deploy these methods especially for clinical applications. We address this issue in the context of DL-based subcortical structures segmentation on T1w brain MRI. We compare two OOD detection frameworks equipping DL segmentation models, Maximum Softmax Probability and Deterministic Uncertainty Method, and demonstrate the superiority of the latter which allows a robust and versatile identification of artifacts in images.Introduction
Deep Learning (DL) models are presently the gold standard for medical image segmentation. However, their performance may drastically drop in the presence of characteristics in test images not present in the training set. The automatic detection of these Out-Of-Distribution (OOD) inputs is the key to prevent the silent failure of DL models, especially when the visual inspection of the input is not systematically carried out1. For MRI segmentation, a wide range of covariables can perturbate a DL model: noise, artifacts or MR sequence parameters.Deterministic Uncertainty Methods (DUM)2 are novel and promising techniques for OOD detection. They propose to analyze the intermediate activations of a trained segmentation DL model to detect OOD inputs. In a previous study, we demonstrated that DUM achieved high OOD detection performance on a task of Multiple Sclerosis lesions segmentation in T2-weighted FLAIR MRI3. To evaluate the generalization capability of this technique, we propose to evaluate DUM in the context of automatic subcortical structures segmentation. We focus our results on the hippocampus and thalamus structures segmentation from T1-weighted MR brain scans of healthy subjects.
Methods
For this work, we used the IXI dataset (https://brain-development.org/ixi-dataset/) composed of 581 healthy T1-weighted brain MRI. For each scan, we used FastSurfer in order to obtain a parcellation of the brain composed of 95 structural classes4, which we used to isolate 2 subcortical regions: the hippocampus and thalamus (Fig. 1). We then split the dataset into a training set (381 subjects) and a testing set (200 subjects). As these test images shared the same origin as the training images, we referred to them as in-distribution (ID) test images. For each ID test image, 7 synthetic OOD images were obtained by using the TorchiO’s library5 transformations: Downsample, Bias, Ghost, Motion, Gaussian Noise, Scale and Spikes (Fig. 2). These OOD images are representative of common MRI artifacts that can be encountered in clinical practice.On the training set, we trained 2 Attention U-Nets 3D6 to respectively segment the hippocampus and thalamus. We then explored two ways of detecting OOD inputs from the trained models: Maximum Softmax Probability (M1) and DUM (M2).
M1. Maximum Softmax Probability7 (MSP) proposes to analyze the output probabilities of the segmentation model to detect OOD. Allegedly, OOD images should yield to lower probabilities than ID images, which should allow for their detection. To implement this method, we retrieved the MSP for each voxel, corresponding to the probability of the predicted class. We then computed the mean MSP across the MRI volume to get a single conformity score for each image.
M2. Deterministic Uncertainty Method (DUM) aims at detecting OOD images based on the intermediate activations of a trained segmentation model. We implement DUM as follows 8: for a given query image, we first gathered the activations F of the penultimate layer of the trained segmentation model. F corresponds to a 4-dimensional array of shape N×H×W×D, H×W×D being the dimension of the 3D MRI and N being the number of features, which is 32 in our DL model. We then computed the Singular Value Decomposition (SVD) of F, and defined its spectral signature as the vector of singular values. The final conformity score corresponds to the minimum euclidean distance between the spectral signature of the test image, and the signatures of all training images. The higher the distance, the more abnormal the input.
Our evaluation protocol was two-fold. First, we evaluated the segmentation quality of the subcortical structures using the Dice score (DSC) between the predictions and the FastSurfer parcellation. Second, we evaluated the ability to distinguish between ID and OOD images using both MSP and DUM. We defined OOD detection as a binary classification problem, where ID test images and OOD images were respectively negative and positive samples. We then computed the AUROC score for each type of MRI artifact by comparing the conformity scores obtained for ID and OOD data.
Results
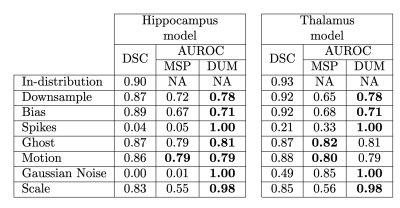
Segmentation and OOD detection performances are presented in Fig. 3. DSC scores decrease on OOD data, yet this drop is only catastrophic for two types of artifacts: Spikes and Gaussian Noise. For these types, only the DUM approach allows a robust identification of the OOD cases, as indicated by the AUROC score of 1.0. Overall, DUM surpasses MSP on 6 out of 7 OOD datasets and on 5 out of 7 for the hippocampus and thalamus segmentation task respectively.Discussion
Overall, DUM surpasses MSP on the task of OOD detection, for both the hippocampus and thalamus models. In cases where the segmentation quality drastically decreases, i.e. Spikes and Gaussian Noise artifacts, DUM allows a perfect identification of non-conform images, which would prevent poor-decision making based on the DL model predictions. Additionally, such Quality Control is easy to implement and computationally efficient as it only requires a trained segmentation model.Conclusion
We compared the performance of two Out-of-Distribution detection frameworks equipping DL segmentation models. Experiments on a set of 7 synthetic MRI artifacts and on 2 subcortical structures segmentation tasks show the superiority of the DUM approach. This confirms the robustness and the versatility of DUM for the identification of non-conform MRIs.Acknowledgements
BL, AT, SD are employees of the Pixyl company. MD and FF serve on Pixyl scientific advisory board.References
1. Jingkang, Y. Zhou, K. Li, Y. and Liu, Z., 2021. Generalized out-of-distribution detection: A survey. arXiv preprint arXiv:2110.11334.
2. Postels, J. Segù, M. Sun, T. Sieber, LD. Van Gool, L. Yu, F. and Tombari F., 2022. Proceedings of the 39th International Conference on Machine Learning, PMLR 162:17870-909.
3. Lambert B., Forbes F., Doyle S., Tucholka A. and Dojat, M., 2022. Improving Uncertainty-based out-of-distribution detection for medical image segmentation, arxiv preprint (2022).
4. Henschel L., Conjeti S., Estrada S., Diers K., Fischl B. and Reuter M., 2020. Fastsurfer-a fast and accurate deep learning based neuroimaging pipeline. NeuroImage, 219, p.117012.
5. Pérez-García F., Sparks R. and Ourselin, S. TorchIO: a Python library for efficient loading, preprocessing, augmentation and patch-based sampling of medical images in deep learning. Computer Methods and Programs in Biomedicine 208 (2021): 106236.
6. Ozan O. et al. Attention u-net: Learning where to look for the pancreas. arXiv preprint arXiv:1804.03999 (2018).
7. Hendrycks D. and Gimpel K., 2016. A baseline for detecting misclassified and out-of-distribution examples in neural networks. arXiv preprint arXiv:1610.02136.
8. Karimi D. and Gholipour A., 2020. Improving calibration and out-of-distribution detection in medical image segmentation with convolutional neural networks. arXiv preprint arXiv:2004.06569.
Figures