3302
Laminar effective connectivity of the default mode network assessed using 7T fMRI1Electrical and Computer Engineering, Auburn University, Auburn, AL, United States
Synopsis
Keywords: Data Analysis, High-Field MRI, Effective connectivity
High-resolution fMRI presents opportunities in the study of fine-scale functional architecture, such as directional interactions between cortical layers. The Default Mode Network (DMN) is a major network in the brain associated with many social behaviors, however, little is known about the feed-forward and feedback information flow between different layers in regions of the DMN. We used high-resolution fMRI combined with effective connectivity to investigate the layer-to-layer interactions of regions in the DMN including medial prefrontal cortex, posterior cingulate cortex, and inferior parietal cortex. The results suggest that the middle layers of PCC are dominant in information flow within the DMN.
Introduction
High-resolution fMRI, having submillimeter voxel dimensions, promises to be an invaluable tool to investigate laminar processing and neural-specific changes across layers in the human brain. The DMN consists of a set of brain regions that are activate in resting subjects1 and are sensitive to psychiatric abnormalities. However, the relationship of cytoarchitectonic layers and functional organization in the DMN is still not fully understood. In a previous study, Huber et al.2 make predictions about layer-specific directionality of neural information flow in the DMN using functional connectivity (FC) based on our prior assumptions about which layers act as inputs and outputs. In this work, we used high-resolution fMRI at 7T in 16 subjects combined with effective connectivity (EC) to explicitly study the directionality of connections between three important regions in the DMN, namely mPFC, PCC and IPC.Methods
Sixteen subjects were recruited for this study. All subjects were scanned on a 7T Siemens MAGNETOM MRI scanner using 32-channel head coil at the Auburn University MRI Research Center. High resolution T1 weighted MPRAGE scan was acquired with 256 slices, voxel size=0.6 mm3, TR/TE 2200/2.8ms, flip angle=7◦. Resting state fMRI multiband EPI scans were acquired with a slice acceleration factor of 3, TR/TE=1500/31ms, no. of volumes=200, slice thickness=0.84x0.84x3.01mm, FOV=260x260x45. Pre-processing of fMRI data involved removal of first five timepoints, slice time correction, realignment, linear detrending, regressing out mean time series from CSF, WM and six head motion parameters. High resolution T1 data was preprocessed and cortical thickness was estimated using Freesurfer pipeline [6,7]. Laminar profiles of 6 layers were delineated at fixed distance from the pial surface, at a depth of 16%,32%,48%,64%, 80% and 96%. Preprocessed fMRI volumes were aligned to the intermediate laminar surfaces using boundary-based affine registration and the registered images were manually checked. DMN regions mPFC, PCC and IPC were obtained from the Desikan Killiany atlas parcellations8.Mean timeseries of these regions were extracted for each of the 6 laminar layers and were deconvolved using a blind HRF deconvolution algorithm 5. Effective (EC) connectivity was calculated between cortical layers of the three DMN regions by implementing Granger Causality (GC)7 on their respective time series.Based on Huber et al.2, we hypothesize that the EC between the middle layers of PCC and the deeper layers of mPFC/IPC will be higher than the other combinations (such as the connectivity from the superficial/deeper layers of PCC to all the layers in mPFC/IPC). We also test the hypothesis that the superficial layer of mPFC drives activity in the deeper layer of IPC.
Results
Figures 1, 2 and 3 show the significant results of the t-test for the directional influence from mPFC to PCC, PCC to IPC and mPFC to IPC. These results confirm hypotheses developed by others based on invasive studies in animals.9Discussion
We can see in Fig. 1 that the connectivity from deeper layer of mPFC to middle layers of PCC is statistically higher than all the connections from all the layers of mPFC to deeper layer of PCC (both L5 and L6). This means there is a feedback pathway from mPFC to PCC. Also in figure 2, we can also see that the connectivity from middle layers (L3/4) of PCC to the deeper of IPC is also statistically higher than the connectivity from superficial layers (L1/2) and deeper layer (L5) of PCC to all the layers of IPC. These results strengthen the hypothesis that PCC is feed-forward while the other two regions are feedback.In figure 3, the t-test also shows that the connectivity from superficial layer of mPFC to deeper layer of IPC is higher than the connectivity from the deeper layer of mPFC to all the layers of IPC. This result indicates that the feed-forward pathway from the superficial layer of mPFC to the deep layer of IPC.
Conclusion
We employed the multivariate autoregressive model to noninvasively infer the layer-specific functional architecture of the DMN. The fact that we are able to infer these laminar specific connections noninvasively in humans opens new possibilities for more fine-grained investigation of the human brain’s functional architecture.Acknowledgements
No acknowledgement found.References
[1] Li, W., Mai, X. and Liu, C., 2014. The default mode network and social understanding of others: what do brain connectivity studies tell us. Frontiers in human neuroscience, p.74.
[2] Huber, L., Finn, E.S., Chai, Y., Goebel, R., Stirnberg, R., Stöcker, T., Marrett, S., Uludag, K., Kim, S.G., Han, S. and Bandettini, P.A., 2021. Layer-dependent functional connectivity methods. Progress in Neurobiology, 207, p.101835.
[3] Fischl, B., 2012. FreeSurfer. Neuroimage, 62(2), pp.774-781.
[4] Waehnert MD, Dinse J, Weiss M, Streicher MN, Waehnert P, Geyer S, Turner R, Bazin PL. Anatomically motivated modeling of cortical laminae. Neuroimage. 2014 Jun;93 Pt 2:210-20. doi: 10.1016/j.neuroimage.2013.03.078. Epub 2013 Apr 16. PMID: 23603284.
[5]. Wu, G.R., Liao, W., Stramaglia, S., Ding, J.R., Chen, H. and Marinazzo, D., 2013. A blind deconvolution approach to recover effective connectivity brain networks from resting state fMRI data. Medical image analysis, 17(3), pp.365-374.
[6] Greve, D.N. and Fischl, B., 2009. Accurate and robust brain image alignment using boundary-based registration. Neuroimage, 48(1), pp.63-72.
[7] Deshpande, G. and Wang, Y., 2022. Noninvasive Characterization of Functional Pathways in Layer-Specific Microcircuits of the Human Brain Using 7T fMRI. Brain Sciences, 12(10), p.1361.
[8] Desikan, R.S., Ségonne, F., Fischl, B., Quinn, B.T., Dickerson, B.C., Blacker, D., Buckner, R.L., Dale, A.M., Maguire, R.P., Hyman, B.T. and Albert, M.S., 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), pp.968-980.
[9] Lawrence, S.J., Formisano, E., Muckli, L. and de Lange, F.P., 2019. Laminar fMRI: Applications for cognitive neuroscience. Neuroimage, 197, pp.785-791.
Figures
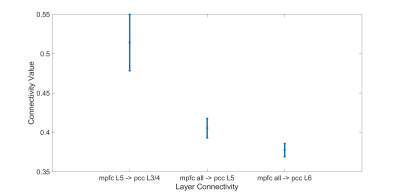
Figure 1. Significant EC between mPFC and PCC. The test shows that the connectivity from deeper layer (L5) of mPFC to middle layers (L3/4) of PCC is higher than the other.connectivity from all the layers of mPFC to deeper layers of PCC (p<0.05 corrected).
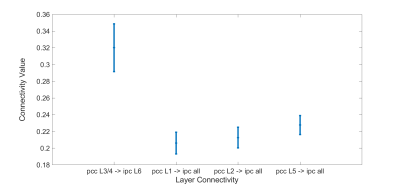
Figure 2. Significant EC from PCC to IPC. The test shows that the connectivity from middle layers (L3/4) of PCC to deeper layer (L6) of IPC is higher than the other connectivity from superficial layers (L1/2) of PCC and deeper layer (L5) of PCC to all the layers of IPC (p<0.05 corrected).
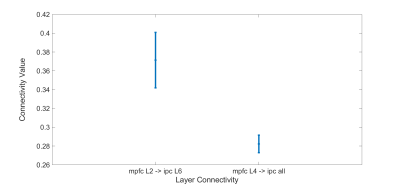
Figure 3. Significant EC from MPFC to IPC. The test shows that the connectivity from superficial layer (L2) of mPFC to deeper layer (L6) of IPC is higher than the connectivity from middle layer of mPFC to all the layers of IPC (p<0.05 corrected).