3295
Whole-Brain Sub-Millimeter Resolution fMRI using 3D EPI Accelerated with Temporal Random Walk1Department of Computer Engineering, Chonnam National University, Gwangju, Korea, Republic of, 2Advanced MRI Technologies, Sebastopol, CA, United States, 3University of California, Berkeley, Berkeley, CA, United States, 4Siemens Medical Solutions USA, Inc, Berkeley, CA, United States
Synopsis
Keywords: Data Acquisition, Brain
There are significant benefits to segmented 3D EPI fMRI acquisitions, which acquires high spatial and temporal resolution across the whole brain to better understand brain neuronal activity. This can be achieved through accelerated 3D EPI imaging, which provides rapid whole-brain coverage at the cost of SNR efficiency resulting from frame-by-frame reconstruction. Here, we utilize temporal information for whole-brain coverage on 8-fold accelerated 7T fMRI acquisition without altering the subsequent fMRI results.Introduction
Ultra-high field increases fMRI signal-to-noise ratio (SNR) and sensitivity to BOLD contrast, making it possible to detect brain activation areas with sub-millimeter resolution, which has become the primary neuroscience research tool for mapping the brain activities across thin cortical layers [1-3]. Particularly, neuroscientists have developed an array of methods capable of looking at whole-brain functional activity and connectivity analysis [4-6].The purpose of this study is to investigate the feasibility of whole-brain 3D EPI fMRI at 0.75mm and 0.64mm isotropic resolutions on the Next Generation 7T scanner using temporal random walk sampling pattern. Experimental studies confirm: 1) leveraging temporal information in fMRI reconstruction provides a high SNR efficiency across time and 2) the resulting high tSNR leads to increased BOLD activations.
Methods
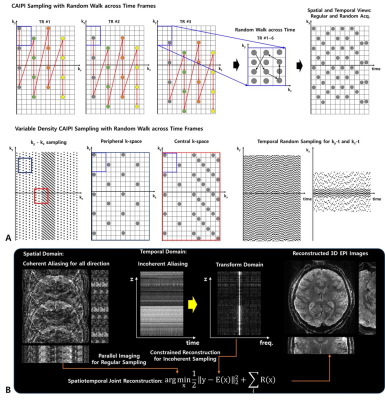
Pulse Sequence and Sampling Pattern: We used multi-shot based segmented 3D EPI sequence for data acquisition to achieve reduced off-resonance artifacts and T2*-blurring, and shorter TEs. The 3D spatial encoding employed variable density CAIPIRINHA (VD-CAIPI) sampling pattern by segmenting kz-space with variable width, in which the central kz-space is sampled at the Nyquist rate while the outer kz-space is regularly under-sampled based on the CAIPI pattern [7,8]. Additionally, as depicted in Figure 1, the time-wise spatial random blips allow extra spatial encoding across time in an incoherently complementary manner within the EPI framework while keeping the coherence on the spatial axes without changing the EPI blip size, resulting in random undersampled spatiotemporal data structure (Fig 1A).Data Acquisition: All fMRI data were collected on the next generation 7T scanner (Siemens Healthcare, Erlangen, Germany) equipped with a 200 mT/m, 900 T/m/s gradient system and a 64-channel head coil. The imaging parameters are: FOV=144x189x132mm3, TR/TE=5s/23ms, FA=18o, resolution=0.64mm and 0.75mm isotropic, number of segments=2, PF=6/8 (phase), and 8-fold acceleration (RyxRz=2x4). As a competing method, we used skipped CAIPI 3D EPI [9], and the image parameters were matched with our sequence.
Reconstruction: All images acquired using VD-CAIP + Random-Walk sampling pattern were reconstructed by leveraging temporal information during reconstruction that imposes dynamic priors (sparse and low rank) along the temporal direction with data consistency (Fig 1B), while images acquired using skipped CAIPI were reconstructed using vendor-preinstalled GRAPPA implementation.
Task and Data Processing: For the stimulation, 1 run with 10s blank screen, 15s on /15s off blocks of flashing checkerboard stimuli were acquired using PsychoPy and a 7T-compatible video projector (VPixx Technologies, Inc). Functional analyses were performed with an afni_proc.py script in AFNI. No smoothing was applied and t-statistics were thresholded at p<0.01, spatially and temporal autocorrelation corrected.
Results and Conclusions
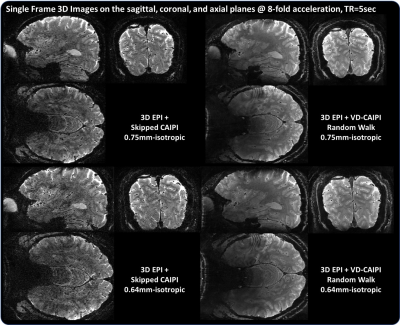
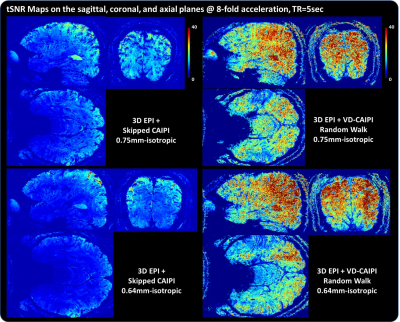
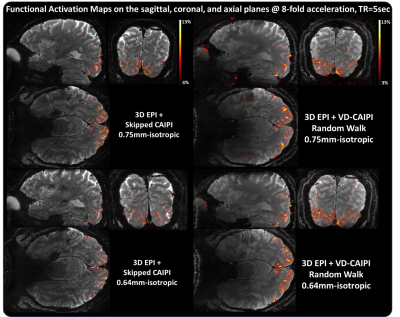
Figure 2 shows whole-brain 3D images in the sagittal, coronal, and axial planes acquired with 0.75 and 0.64 isotropic resolutions. The proposed method effectively suppresses image artifacts resulting from temporal leveraging, leading to clear depiction of brain structures compared to skipped-CAIPI. Figure 3 shows the corresponding tSNR maps. The temporal leverage significantly increases the tSNR values by minimizing a trade-off between SNR and accuracy. Figure 4 shows functional activation maps overlaid on the average image. Note that the proposed method shows much higher BOLD sensitivity in the vicinity of gray matter compared to the competing method. We find that 1) a high SNR efficiency across time leads to increased BOLD sensitivity and 2) the segmented 3D EPI coupled with a random sampling scheme can be advantageous for sub-millimeter resolution fMRI smaller than 0.7mm and whole-brain layer fMRI approach.Acknowledgements
This project is supported in part by the NIH BRAIN Initiative (R01MH111444, U01EB025162), 1R44MH129278 (to Feinberg), and NRF/MSIT No. 2021R1C1C1013603 (to Park).References
[1] Huber L, Ivanov D, Handwerker DA, Marrett S, Guidi M, Uludağ K, Bandettini PA, Poser BA. Techniques for blood volume fMRI with VASO: From low-resolution mapping towards sub-millimeter layer-dependent applications. Neuroimage. 2018; 164:131-143.
[2] Goense J, Merkle H, Logothetis NK. High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses. Neuron. 2012; 76(3):629-639.
[3] Kashyap S, Ivanov D, Havlicek M, Sengupta S, Poser BA, Uludağ K. Resolving laminar activation in human V1 using ultra-high spatial resolution fMRI at 7T. Scientific reports. 2018; 8(1):1-1.
[4] Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Uğurbil K. Multiband multislice GE‐EPI at 7 tesla, with 16‐fold acceleration using partial parallel imaging with application to high spatial and temporal whole‐brain fMRI. Magn Reson Med. 2010; 63(5):1144-53.
[5] Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, Miller KL, Ugurbil K, Yacoub E. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PloS one. 2010;5(12):e15710.
[6] Poser BA, Koopmans PJ, Witzel T, Wald LL, Barth M. Three dimensional echo-planar imaging at 7 Tesla. Neuroimage. 2010; 51(1):261-266.
[7] Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi‐slice imaging. Magn Reson Med. 2005; 53(3):684-91.
[8] Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reson Med. 2006; 55(3):549-556.
[9] Stirnberg R, Stöcker T. Segmented K‐space blipped‐controlled aliasing in parallel imaging for high spatiotemporal resolution EPI. Magn Reson Med. 2021; 85(3):1540-51
Figures