3293
Feasibility of routine brain imaging at 0.55T: initial experience in a clinical workflow1Department of Radiology, University of Michigan, Ann Arbor, MI, United States, 2Siemens Medical Solutions USA Inc., Houston, TX, United States
Synopsis
Keywords: Low-Field MRI, Brain
The purpose of this study is to assess the quality of brain imaging studies performed on an FDA-approved commercial 0.55T MRI system, and to provide information about the feasibility of using this scanner in a clinical workflow. The image quality of 1356 image series (378 in healthy subjects, 978 in patients) was independently rated by two neuroradiologists. While images from T1w SPACE, DWI/ADC, FLAIR, SWI, and T2w TSE sequences received acceptable image quality scores, contrast-enhanced T1w TSE sequence received lower, although acceptable, average scores. Our results suggest that neurological MRI studies for some indications can be performed at 0.55T.Introduction
The goal of this work is to assess the quality of routine brain imaging in both healthy subjects and patients on an FDA-approved 0.55T MRI system, and to determine the feasibility of using this scanner in a clinical workflow. Currently, most clinical MRI scanners are higher field (1.5T and 3T) systems. Lower field MRI systems have recently been introduced to potentially lower the cost of MR imaging and improve accessibility. In addition to reduced cost, some artifacts may be reduced at lower field strengths, thus improving the image quality near air-tissue interfaces or metallic implants1,2. Previous work on 0.55T MRI systems has shown the feasibility of its use in interventional, diagnostic brain imaging3,4, and in the application of specific neuroimaging protocols, such as MR Fingerprinting and fMRI5,6. However, the image quality of routine brain imaging sequences acquired from the wide range of subjects on this whole body 0.55T system has not yet been assessed. Thus, this study aimed to assess the image quality of clinical brain imaging sequences on a 0.55T scanner in healthy subjects and patients with the goal of determining the feasibility of using this system for patients undergoing our routine brain imaging protocol.Methods
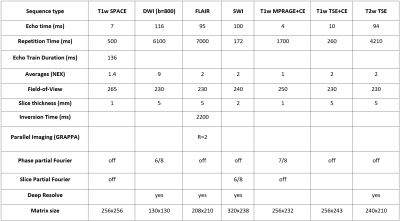
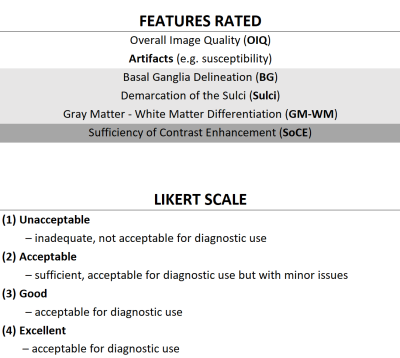
In this IRB-approved study, clinical brain sequences were acquired in healthy subjects and patients with indications suitable for our routine brain imaging protocol on an FDA-approved commercial 0.55T MRI system (MAGNETOM Free.Max, Siemens Healthineers, Erlangen, Germany) from February to July 2022. The protocol for healthy subjects was comprised of T1w SPACE, DWI/ADC, FLAIR, SWI (magnitude and phase), and T2w TSE sequences. In addition to the above-listed sequences, the protocol for patients included unenhanced and contrast-enhanced (CE) T1w MPRAGE and T1w TSE. Sequence parameters are shown in Figure 1. The standard 12-channel head coil was used for all subjects. Images from healthy subjects and patients were rated separately and independently by two neuroradiologists for overall image quality and specific image features on a four-point Likert scale (Figure 2). For each sequence, the mean and standard deviation of the ratings and the fraction of images with acceptable ratings were calculated for each feature and each reader. To determine inter-reader reliability, the percentage agreement between readers on the images to be of acceptable (≥2), or unacceptable (<2) quality was calculated. Finally, readers were asked whether the clinical question could be answered for each patient.Results
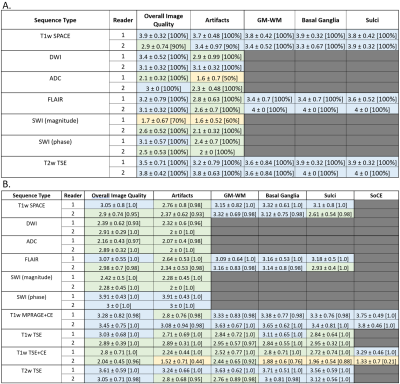
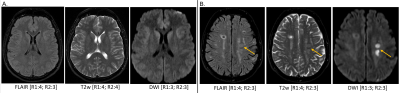
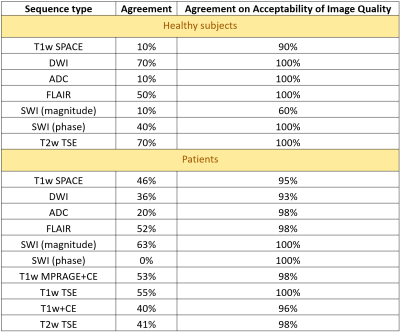
Overall, 378 image series from 10 healthy subjects (3F; mean age 39.9 years, range 24-65 years) and 978 image series from 44 patients (26F; mean age 55.5 years, range 20-94 years) were acquired at 0.55T. Image quality ratings are shown in Figure 3. In healthy subjects, more than 90% of all features on all sequences were rated as acceptable (≥2), except for overall image quality and artifacts on SWI from Reader 1. As for patients, more than 90% of all features in each of the sequences were rated acceptable, except for artifacts and sufficiency of contrast enhancement of CE T1w TSE from Reader 2. Patient studies performed on 0.55T enabled the readers to answer the clinical question in all scanned patients. The following findings were made: normal brain (n=15), non-specific T2/FLAIR white matter hyperintensities (n=11), age-related cerebral volume loss (n=6), subdural hemorrhage (n=3), metastatic disease (n=3), partially empty sella turcica (n=2), embolic disease (n=2), acute stroke (n=1), subacute stroke (n=1), chronic stroke (n=1), lacunar infarct (n=1), arachnoid cyst (n=1), meningioma (n=1), multiple sclerosis (n=1), postsurgical hygroma (n=1), Rathke’s cleft cyst (n=1), Potts puffy tumor (n=1), diffuse axonal injury (n=1), ventriculomegaly (n=1). Example images are shown in Figure 4. Despite high variability of agreement between the individual scores of the readers (10%-90%), there was a high degree of consensus on acceptable (ratings ≥2) or unacceptable (ratings <2) image quality ratings (Figure 5).Discussion
Lower cost, low-field MR scanners may enable improved access to MRI. While the whole-body 0.55T system assessed in this work could be used to image any part of the body, it is imperative that images acquired using the routine brain protocol are of diagnostic quality, as brain indications for MRI are common. Almost all images acquired in healthy subjects and patients received scores indicating that they were acceptable for clinical use, allowing the clinical question to be answered for all scanned patients. These sequences form the basis of several common neurological MRI protocols (e.g., routine brain, brain tumor, and brain metastases protocols), which based on these results could be performed at 0.55T. While the SWI (magnitude) sequence was scored lower than other sequences in healthy subjects by Reader 1, all images received acceptable overall image quality scores in the patient population from both readers, indicating that lower image quality and acceptable diagnostic efficacy may be uncoupled. However, many images from T1w TSE+CE sequence for Reader 2 had average ratings for certain features in the unacceptable range, indicating that further sequence optimization or alternative imaging strategies might be required.Conclusion
Acceptable quality clinical brain images can be collected using our routine brain imaging protocol on a commercial 0.55T MRI system in healthy subjects and patients, indicating that the routine brain imaging protocol may be deployed on this system in the clinical workflow.Acknowledgements
Research support from Siemens HealthineersReferences
[1] Breit, Hanns-Christian, Jan Vosshenrich, Thilo Rusche, Michael Bach, and Dorothee Harder. "Improving the Assessment of the Postoperative Spine with 0.55 T MRI: A Case Report."
[2] Pogarell, Tobias, Matthias S. May, Armin M. Nagel, Michael Uder, and Rafael Heiss. "Imaging of the musculoskeletal system using low-field magnetic resonance imaging." Der Radiologe (2022).
[3] Campbell-Washburn AE, Ramasawmy R, Restivo MC, Bhattacharya I, Basar B, Herzka DA, Hansen MS, Rogers T, Bandettini WP, McGuirt DR, Mancini C, Grodzki D, Schneider R, Majeed W, Bhat H, Xue H, Moss J, Malayeri AA, Jones EC, Koretsky AP, Kellman P, Chen MY, Lederman RJ, Balaban RS. Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology. 2019 Nov;293(2):384-393. doi:10.1148/radiol.2019190452.
[4] Runge VM, Heverhagen JT. Advocating the Development of Next-Generation, Advanced-Design Low-Field Magnetic Resonance Systems. Invest Radiol.2020 Dec;55(12):747-753. doi: 10.1097/RLI.0000000000000703. PMID: 33156083.
[5] Campbell-Washburn AE, Jiang Y, Körzdörfer G, Nittka M, Griswold MA. Feasibility of MR fingerprinting using a high-performance 0.55 T MRI system. MagnReson Imaging. 2021 Sep; 81:88-93. doi: 10.1016/j.mri.2021.06.002. Epub 2021 Jun 8. PMID: 34116134.
[6] Wang Y, van Gelderen P, de Zwart JA, Campbell-Washburn AE, Duyn JH. FMRI based on transition-band balanced SSFP in comparison with EPI on a high-performance 0.55 T scanner. Magn Reson Med. 2021 Jun;85(6):3196-3210. doi: 10.1002/mrm.28657. Epub 2021 Jan 21. PMID: 33480108; PMCID: PMC7 904622.
Figures