3292
Spectrum of Brain Injury in COVID-19 Acute Respiratory Distress Syndrome: A Systematic Review of Neuroimaging Findings
Rachel Wagner1,2, Michael T Jurkiewicz2,3, Jennifer Chen4,5, Angela Jerath6,7, Marat Slessarev2,5,8, and Udunna Anazodo2,9
1Neuroscience, Western University, London, ON, Canada, 2Lawson Health Research Institute, London, ON, Canada, 3Medical Imaging, London Health Sciences Centre, London, ON, Canada, 4Critical Care Medicine, Western University, London, ON, Canada, 5Western Institute for Neuroscience, Western University, London, ON, Canada, 6Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 7Evaluative Clinical Sciences, Schulich Heart Program, Sunnybrook Research Institute, Toronto, ON, Canada, 8Critical Care Medicine, Schulich School of Medicine & Dentistry, Western University, London, ON, Canada, 9Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada
1Neuroscience, Western University, London, ON, Canada, 2Lawson Health Research Institute, London, ON, Canada, 3Medical Imaging, London Health Sciences Centre, London, ON, Canada, 4Critical Care Medicine, Western University, London, ON, Canada, 5Western Institute for Neuroscience, Western University, London, ON, Canada, 6Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 7Evaluative Clinical Sciences, Schulich Heart Program, Sunnybrook Research Institute, Toronto, ON, Canada, 8Critical Care Medicine, Schulich School of Medicine & Dentistry, Western University, London, ON, Canada, 9Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada
Synopsis
Keywords: Infectious disease, COVID-19, Neuroscience
While most COVID-19 patients present with mild symptoms, many of those hospitalized with severe infection develop acute respiratory distress syndrome (ARDS)1 and frequently report central nervous system dysfunction2. While previous research has characterized clinical symptomatology, investigation regarding brain changes remains limited. To address this gap, this review summarized brain injuries from 372 COVID-19-ARDS and 370 ARDS patients and pooled findings. COVID-19-ARDS patients presented an unusual pattern of injury preferentially targeting white matter, suggesting impact to brain connectivity. The long-term consequences of white matter injury in COVID-19-ARDS should be taken into account in devising policies for managing patient health.Introduction
While the majority of COVID-19 patients present with mild respiratory symptoms, many of those hospitalized with severe infection develop a form of respiratory failure known as acute respiratory distress syndrome (ARDS), typically requiring Intensive Care Unit (ICU) admission and supplemental oxygenation1. Previous research has implicated central nervous system dysfunction in the pathophysiology and sequelae of COVID-19-ARDS, including delirium, cognitive deficits, and psychiatric impairments2. Of particular concern, changes in brain structure and function have been increasingly reported in patients, indicating heightened risk for poor functional outcomes. While many studies have characterized the clinical symptomatology of COVID-19-ARDS, comprehensive investigation regarding neuroimaging findings is limited. To address this gap in the literature, this review summarized the type, prevalence, and location of neuroimaging findings identified using MRI, CT, or PET from COVID-19-ARDS patients and to compare them to those from non-COVID-19 ARDS patients in order to inform healthcare practitioners and future research.Methods
We employed a pre-determined search strategy within MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), WHO International Clinical Trials Registry Platform (ICTRP), and Clinicaltrials.gov databases to identify studies with MRI, CT, or PET neuroimaging data in adult patients with COVID-19-ARDS or non-COVID-19-ARDS. Two reviewers independently screened and extracted data using a standardized extraction tool. Studies were assessed for bias using the Newcastle-Ottawa Scale and GRADE methodology. The protocol for this systematic review was pre-registered on PROSPERO. Neuroimaging findings were stratified by imaging modality and descriptive statistics were used to illustrate findings in a narrative fashion. A frequency map of reported brain outcomes was generated to the corresponding brain regions in Matlab and Freesurfer to perform a joint registration‐segmentation procedure to an internal FreeSurfer volumetric space3.Results
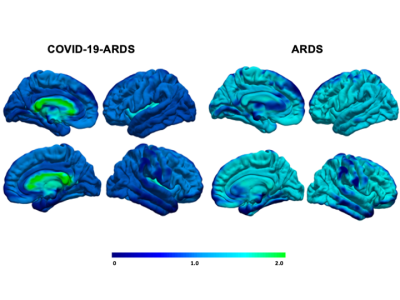
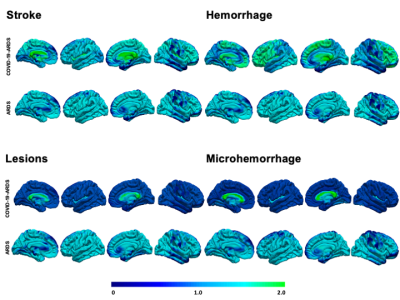
We included 28 studies comprising 372 COVID-19-ARDS and 370 non-COVID-19-ARDS patients. The COVID-19-ARDS cohort was older (60.7 vs. 52 years) and had a lower proportion of females (26.3% vs 46.8%), but there were no differences in the severity of ARDS between groups, as indicated by similar PaO2/FiO2 ratios (118.4 vs. 106.5 mmHg). While incidence of ischemic stroke and inflammatory markers were also similar between groups, levels of fibrinogen were elevated in COVID-19-ARDS (6.3 vs 3.2 g/L). The most common neuroimaging findings were intracerebral hemorrhages (42.8% vs. 18.9% in COVID-19-ARDS and non-COVID-19-ARDS respectively), predominantly intraparenchymal in COVID-19-ARDS (29.3% vs. 10.9%). White matter abnormalities were markedly increased in COVID-19-ARDS (48.3% vs. 3.1%), as were microhemorrhages with a predilection for the corpus callosum (24.31% and 1.2%). The most common neurocognitive complications reported during hospital stay included impaired consciousness (29.7% vs. 0.8%), oculomotor disturbances (10.1% vs 15.3%), and gait instability (9.2% vs 0.0%).Discussion
While the prevalence and types of brain injuries were similar between groups, the location of injury differed in COVID-19-ARDS, presenting an unusual pattern preferentially targeting deep white matter, in contrast to a greater cortical distribution in non-COVID-19-ARDS. This may suggest a unique pathophysiological component linked to COVID-19, potentially implicating distinct functional consequences. White matter tissue composes over 40% of the total brain volume and is critical for motor neurotranmission and executive functioning, implicating significant risk for post-acute disability4,5. This may be further highlighted by the increased incidence of gait instability and executive and sensorimotor impairments reported in the COVID-19-ARDS group relative to ARDS. Similar patterns have been reported in patients receiving extracorporeal membrane oxygenation (ECMO) and in high-altitude sickness, indicating vulnerability to hypoxic damage3,6. In light of similar PaO2/FiO2 ratios, this pattern may be linked to exacerbation of thrombotic response increasing damage susceptibility, as suggested by heightened fibrinogen levels in COVID-19-ARDS6. Several mechanisms have been proposed to explain the neurological impact contributed by COVID-19 infection, including release of inflammatory factors activating macrophages, microglia and astrocytes, systemic hypoxemia due to breakdown of pulmonary surfactant, and endothelial dysfunction favoring a prothrombotic state7,8. Altogether, our results indicate a synergistic effect comprising multiple mechanistic components contributed by both ARDS and severe infection, conferring increased vulnerability for white matter injury.Conclusion
Indicated patterns of white matter injury in COVID-19-ARDS provide an immediate area of concern for researchers and clinicians, as such damage has been linked to gait instability, cognitive and emotional dysfunction and impairments in executive and sensorimotor control8. Our report adds to this growing body of evidence by providing a comprehensive account of the neurologic consequences of COVID-19. The long-term consequences of severe SARS-CoV-2 infection may result in rising incidence of disability-related impairments and should be taken into account in devising policies for managing patient health and public health burden9. Future research should seek to establish the link between observed patterns of brain injury and neurocognitive, psychiatric and functional outcomes to better support this patient population.Acknowledgements
We thank Raymond Confidence, Paulien Moyaert and Meagan Stanley for assisting in conceptual design and workflow.References
- Bain A, Yang W, Shah F, et al. COVID-19 versus Non-COVID-19 Acute Respiratory Distress Syndrome: Comparison of Demographics, Physiologic Parameters, Inflammatory Biomarkers, and Clinical Outcomes. Annals of American Thoracic Society, 2021;18(7):1202-1210
- Hopkins R. O., Suchyta M. R., Beene K, et al. Critical illness acquired brain injury: Neuroimaging and complications for rehabilitation. Rehabilitation Psychology, 2016;61(2):151-164.
- Fischl B, Salat D.H., Busa E., et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 2002;33:341-355.
- Li J, Xiao L, He D, Luo Y, et al. Mechanism of White Matter Injury and Promising Therapeutic Strategies of MSCs After Intracerebral Hemorrhage. Frontiers Aging in Neuroscience, 2021;13.
- Wang Y, Liu G, Hong D, Chen F, et al.White matter injury in ischemic stroke. Progress in Neurobiology, 2021;141:45-60.
- You Q, Yang Y, Hu H. White Matter Hyperintensities and Functional Outcomes in Patients With Cerebral Hemorrhage: A Systematic Review and Meta-Analysis. Frontiers Neurology. 2022;13:820012.
- Lacerte M, Shapshak A, Mesfin F. Hypoxic Brain Injury. 2021. https://www.ncbi.nlm.nih.gov/books/NBK537310/. Accessed November 6th 2022.
- Sui J., Noubouossie D., Gandotra S., Cao, L. Elevated Plasma Fibrinogen Is Associated With Excessive Inflammation and Disease Severity in COVID-19 Patients Frontiers in Cellular and Infection Microbiology, 2021;3.
- Spudich S, Nath A. Nervous system consequences of COVID-19. Science 2022;375:267–269
DOI: https://doi.org/10.58530/2023/3292