3289
Maximization of cucurbit[6]uril hyperpolarized chemical exchange saturation transfer (HyperCEST) in bovine blood at 3.0 T1Chemistry and Materials Science Program, Lakehead University, Thunder Bay, ON, Canada, 2Thunder Bay Regional Health Research Institute, Thunder Bay, ON, Canada, 3Chemistry, Lakehead University, Thunder Bay, ON, Canada, 4Applied Life Science Program, Lakehead University, Thunder Bay, ON, Canada, 5Xemed LCC, Durham, NH, United States, 6Northern Ontario School of Medicine, Thunder Bay, ON, Canada
Synopsis
Keywords: Hyperpolarized MR (Gas), Contrast Agent
Cucurbit[6]uril is a well-studied contrast agent for hyperpolarized 129Xe (HP 129Xe) MRI. Although the in vivobiodistribution of CB6 was measured with hyperpolarized chemical exchange saturation transfer (HyperCEST), there were no previous studies reported for maximization of the HyperCEST effect by optimization of depolarization pre-pulse trains. In the present work, we maximized the CB6 HyperCEST effect in bovine blood, and found for the first time, a HyperCEST depletion of the RBC resonance. In addition, the minimum detectable concentration was found to be four times lower than previously reported.
Introduction
Utilization of MRI for molecular imaging is favourable over other medical imaging modalities for in vivo application due to its absence of ionizing radiation and high spatial resolution1,2. The hyperpolarized (HP) chemical exchange saturation transfer (HyperCEST) effect greatly increases the sensitivity of HP 129Xe MRI. Cucurbit[6]uril is one of the most promising supramolecular agents for HyperCEST molecular imaging. To date, it is the only HyperCEST contrast agent studied in vivo3. However, all previous studies utilized only 3-lobe sinc depolarization pulses with similar flip angles (FA) of 330° and 400° 4,5. No other depolarization pulse trains were examined. In the present work, we continued optimization the HyperCEST effect for CB66 and studied the effect of different depolarization pulses on the CB6 HyperCEST effect in sterile citrated bovine blood and further increased the detectability limit.Experimental method
This study was conducted using a clinical Philips Achieva 3.0T MRI scanner equipped with a custom-built quadrature coil tuned to the 129Xe resonance frequency (35.33 MHz). Naturally abundant 129Xe (~26%) was polarized up to 56% using a XeBox-10E polarizer (Xemed LCC, USA). Spectral data were acquired in vitro using a set-up similar to that used by Norquay et al7. Mixing of sterile bovine blood (Cedarlane, Burlington, CA, USA) and HP 129Xe was performed using an exchange module (Superphobic MicroModule 0.5×1 G680 Contactor; Membrana, North Carolina, USA) and two 10 mL syringes. The blood was manually pumped through the exchange module perpendicular to the 129Xe flow for ~3 sec.Samples of CB6 dissolved in blood (1mM, 0.4mM, 0.25mM, 0.1mM, 25uM, and 10uM ) were prepared by adding a constant volume (1.33 mL) of CB6 in PBS solutions to blood (8.67 mL) in order to get 10 mL samples. The concentrations of CB6 in PBS solutions were 0.075mM, 0.1875mM, 0.375mM, 0.75mM, 1.875mM, 3.75mM, 7.5mM to keep the dilution of blood constant. Samples were drawn into the 10 mL syringe and pumped through the exchange module.
Four different RF saturation pre-pulse trains were used for HyperCEST MRS: 3-lobe sinc (3LS) pulses, sinusoidal (sine) pulses, block pulses, and hypersecant (hypsec) pulses. 0 ppm was set in the middle between the 129Xe plasma peak and 129Xe red blood cells (RBC) peak. The pre-pulse train parameters are shown in the Table 1. Dynamic HyperCEST MRS was performed using the following parameters: TR/TE=10s/0.25ms, receiving bandwidth(BW)=32kHz, 90o excitation rectangular pulse, 2048 sampling data points. Raw data were initially processed in MATLAB 2021b (MathWorks, USA) and further postprocessed in OriginPro 2021b (OriginLab Corp, USA).
Results and discussion
Fig.1 shows HyperCEST spectra of 1mM CB6 solutions in PBS after application of four different depolarization pulse-trains with the same FA of 1200°. The HyperCEST effect was observed after pulses at -70 ppm from the dissolved phase peak. The HyperCEST depletion values were similar (~96%) for 3LS, block and sine pulses. The hypsec pulse showed slightly lower depletion of 90%.Typical HyperCEST depletion spectra of 1mM solutions of CB6 in PBS and bovine blood acquired with sinusoidal pre-pulses are showed in Fig.2. The HyperCEST effect in bovine blood was observed at -86 ppm. The intensity of the HyperCEST effect observed in bovine blood was 12% lower than in PBS and had a higher variability. This can be explained by the presence of other molecules in bovine blood which are able to block the CB6 cavity from interaction with dissolved 129Xe. In addition, the dissolved 129Xe peak in blood was almost twice wider compared to the PBS peak, which can be explained by the existence of the constant chemical exchange of 129Xe between the two different resonances: 129Xe-RBC and 129Xe-plasma.
For the first time, we have observed a HyperCEST effect for both the 129Xe-plasma and 129Xe-RBC pools. This previously unreported phenomenon was achieved by minimal and constant dilution of blood with stock CB6 solutions for all concentrations, which served to maintain the balance and constant chemical exchange between the 129Xe-RBC and 129Xe-plasma pools.
The HyperCEST effect change with CB6 concentration in both the PBS and blood is shown in Fig.3. The maximum HyperCEST effect observed in 1mM solutions of CB6 in blood and PBS was achieved using block and 3ls depolarization pulses, while the sine and hypsec pulses resulted in lower depletions. The most significant drop of HyperCEST in bovine blood (from 83% to 28%) was noticed for the hypsec pulse for concentrations of CB6 lower than 1mM. All other pulses demonstrated a gradual decrease in the HyperCEST effect.
The detectability limit was found to be 0.1 mM of CB6 in blood for all depolarization pulses. The strongest HyperCEST effect of 0.1 mM CB6 in blood was 30% in plasma and 25% in the RBC peak for the block depolarization pulse. The 3LS pulse shape demonstrated a slightly lower (~28%) HyperCEST effect, while the other two pulse shapes showed only a ~14% HyperCEST effect. Overall, the HyperCEST detection limit for all saturation pulses was found to be 4 times lower than previously reported by Hane et al. (0.25mM)4. The results of our work demonstrate substantial optimization of the saturation pre-pulses for future in vivo HyperCEST experiments using a clinical MRI scanner.
Acknowledgements
This study was funded by a Natural Science Engineering Research Council (NSERC) Discovery grant (RGPIN-2017-05359). YS was supported by a MITACS Elevate postdoctoral fellowship (IT25574). VG was supported by an Ontario Trillium Scholarship and an NSERC Returning Student Award. VB was supported by an NSERC Special Fund for Trainees (Ukraine) (RGPIN-2017-05359).
References
1. Schroder L, Lowery TJ, Hilty C, Wemmer DE, Pines A. Molecular Imaging Using a Targeted Magnetic Resonance Hyperpolarized Biosensor. Science (1979). 2006;314(5798):446-449. doi:10.1126/science.1131847
2. Kim SJ, Lee HY. In vivo molecular imaging in preclinical research. Lab Anim Res. 2022;38(1):31. doi:10.1186/s42826-022-00142-3
3. Hane FT, Li T, Smylie P, et al. In vivo detection of cucurbit[6]uril, a hyperpolarized xenon contrast agent for a xenon magnetic resonance imaging biosensor. Sci Rep. 2017;(3):41027. doi:10.1038/srep41027
4. Hane FT, Smylie PS, Li T, et al. HyperCEST detection of cucurbit[6]uril in whole blood using an ultrashort saturation Pre-pulse train. Contrast Media Mol Imaging. 2016;11(4):285-290. doi:10.1002/cmmi.1690
5. McHugh CT, Kelley M, Bryden NJ, Branca RT. In vivo hyperCEST imaging: Experimental considerations for a reliable contrast. Magn Reson Med. 2021;(August):1-10. doi:10.1002/mrm.29032
6. Grynko V, Shepelytskyi Y, Li T, Aalto H, Ruset IC, Albert MS. Saturation pre-pulse train optimization for maximization of cucurbit[6]uril hyperpolarized chemical exchange saturation transfer at 3.0 T. In: Proc.Intl.Soc.Mag.Reson.Med.30. Vol 314. ; 2022:3572. doi:10.1126/science.1131847
7. Norquay G, Leung G, Stewart NJ, Wolber J, Wild JM. 129Xe Chemical Shift in Human Blood and Pulmonary Blood Oxygenation Measurement in Humans Using Hyperpolarized 129Xe NMR. Magn Reson Med. 2017;77:1399-1408. doi:10.1002/mrm.26225Figures
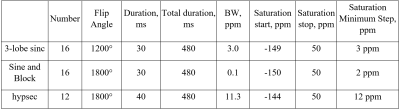
Table 1. Parameters of the depolarization pre-pulse trains.
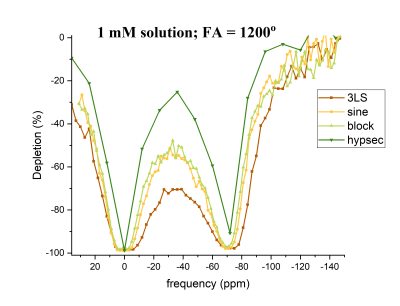
Figure 1. HyperCEST depletion spectra of a 1mM solution of CB6 in PBS after application of 3-lobe sinc, sine, block, hypsec pulses with 1200° flip angles.
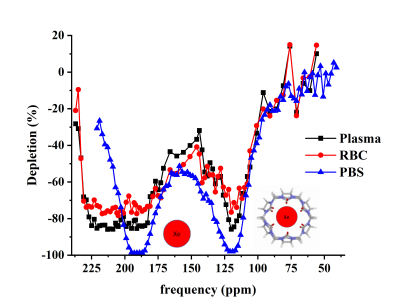
Figure 2. HyperCEST depletion spectra for 1 mM CB6 in blood and PBS after application of a sinusoidal depolarization pulse with 1800º flip angle.
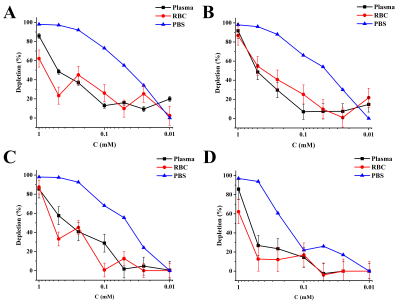
Figure 3. CB6 concentration effect on the HyperCEST depletion in bovine blood after application of A) sinusoidal depolarization pulse with 1800º FA, B) block pre-pulse train of 1800º FA, C) 3-lobe sinc pulse using 1200º FA, and D) 1800º FA hypersecant depolarization pulse.