3286
Demonstration of B1 mapping based RF shimming for CEST imaging at 7T1Advanced imaging research center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Brain repair and Rehabilitation, UCL, London, United Kingdom, 3Philips healthcare, Florida, FL, United States, 4F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, United Kingdom, 5Department of Radiology, Johns Hopkins University, Baltimore, MD, United States, 6Department of Radiology, University Medical Center Utrecht, Utrecht, The Netherlands, Ultrecht, Netherlands, 7Department of Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 8Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
Keywords: CEST & MT, Brain, B1 inhomogeneities
In this work, we sought to evaluate CEST in combination with RF shimming that is based on the modulation of the amplitudes and phases of the RF pulses transmitted by an eight channel 7T MR system.Introduction
High field MRI suffers from transmit field B1+ inhomogeneity because of the shorter wavelength and the attenuation of the RF amplitude due to tissue conductivity (1). In this work, we sought to evaluate CEST in combination with RF shimming that is based on the prospective optimization of the amplitudes and phases of the RF pulses transmitted by an eight channel RF transmit 7T MR system.Methods
CEST data were acquired on four healthy volunteers using a 7T whole-body MRI scanner (Philips Healthcare) equipped with an 8-channel parallel transmission system in accordance with approval from the local ethics committee. All scans were performed using a custom made 8-channel transceiver RF coil as described previously (3).The CEST pulse sequence consisted of a presaturation module of 56 gaussian pulses of 30 ms duration each with 20 ms interpulse delay. Other parameters are as follows: 2D FFE, Cartesian, single-shot TFE acceleration, TR/TE=4.0/1.78 ms, slice thickness 4 mm. The saturation flip angle was either 700 (0.8 µT) aiming at APT or 350 (0.4 µT) degrees aiming at NOE. The off-resonance frequencies varied from +5 ppm to -5 ppm to obtain either a 28-point Z-spectrum (N=2) or a densely-sampled spectrum of 50 points (N=2).
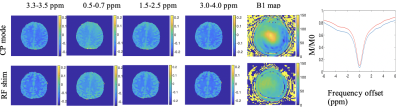
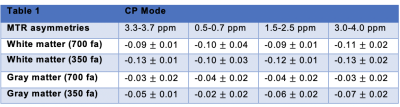
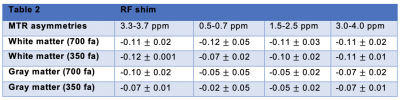
MTR asymmetries were calculated as the difference in signal on either side of the water peak for four frequency offsets ranges: 3.3-3.7 ppm, 0.5-0.7 ppm, 1.5-2.5 ppm and 3.0-4.0 ppm. B0 correction used the minimum of a fitted spline. For each volunteer, Regions-of-interest (ROIs) delimiting white and gray matter were drawn on the anatomical image and then applied on the corresponding MTR asymmetry maps.
For RF shimming we used a volume RF shimming algorithm for amplitude and phase shimming which is fully integrated in the MRI scan software and uses only 2D data from the center slice of a single channel B1+ mapping prescan. The prescan was obtained from all the individual 8-channels based on a 3D AFI protocol as described previously (FA=25, TRs=80, 160 ms) (2). The resulting RF shim (i.e. the optimized amplitudes and phases from each RF channel) was then used in conjuction with the CEST protocol to homogenise the B1+ field for CEST applications. A circularly polarised (CP) mode scan was also used as a reference which uses equal amplitudes (0.1163) for all channels and the phases are 0, 45, 90, 135, 180, 225, 270, 315. For a healthy volunteer an examplary optimal set of RF parameters were: amplitudes: 0.157, 0.128, 0.157, 0.1486, 0.157, 0.157, 0.157, 0.157, phases: -15, 45, 19, 121, 161, 224, 270, 314.
Results and Discussion
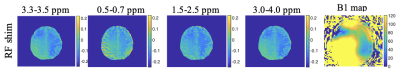
Figure 1 shows the MTR asymmetries of a 28-point Z-spectrum calculated as described above using either the CP mode or the RF shim algorithm. It is evident that the CEST contrast in the images produced using the RF shimming is more homogeneous and displays less artefacts(red arrows) when compared to the ones obtained from CP mode for the 2 irradiation amplitudes we used here. Figure 2 displays the MTR asymmetries obtained with a densely-sampled Z-spectrum for CP mode and RF shimming. While the beneficial effect of RF shimming on the CEST contrast homogeneity is still visible, the produced CEST images appear generally smoother when compared to the ones obtained with an under sampled Z-spectrum due to the higher spectral resolution in the Z-spectra (Figure 2). On another note we used herein a tight fit transceiver array (4) with optimized positioning (see Figure 5 in reference 7)yielding more homogeneous images in general even in the case of CP mode compared to the industrial made coils that utilize separate transmit and receive arrays. Figure 3 shows the effect of a wrongly calculated RF shim in case the RF shimming algorithm did not converge. We observed that it is generally required to perform the RF shimming calculations multiple times and checking the quality of the acquired single channel B1+ maps as well as the calculated RF shim prior to the CEST scans. As it can be seen from our images the obtained CEST maps at high field reflect the B1+ pattern. Thus, it is crucial to prospectively correct for B1+ inhomogeneity for CEST at high field but also to optimise careful the B1+ mapping scan protocol as well as the RF shimming algorithm to obtain artefact free maps and good RF shim results. Finally, Tables 1 and 2 display the values of MTR asymmetries calculated from all the 4 volunteers in white and gray matter for both the irradiation amplitudes used here. According to our results higher irradiation amplitudes are more prone to B1+ artefacts when compared to lower irradiation powers such as 0.4 uT. To conclude we demonstrated that using RF shimming technology at high field for CEST imaging is promising however, it requires careful optimisation of the B1+ maps and RF shim algorithm and quality assurance for correct outcomes.Acknowledgements
This work was funded by Cancer Prevention and Research Institute of Texas (CPRIT) RR180056 and was performed under the rubric of the Advanced Imaging Research Center, UT Southwestern Medical CenterReferences
1. Bottomley P, Andrews E. RF magnetic field penetration, phase shift and power dissipation in biological tissue: Implications for NMR imaging. Phys Med Biol. 1978;23:630–643.
2. Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med. 2007 Jan;57(1):192-200. doi: 10.1002/mrm.21120. PMID: 17191242.
3. Zhang, B., Zaki, A, Dimitrov, I, Thomas B.,Pfommer, A & Henning A. Robust decouplying of a 7 Tesla 8-channel loop array for head and cervical spinal cord imaging. ISMRM & SMRT annual meeting
4. Avdievich, N.; Hoffmann, J.; Shajan, G.; Pfrommer, A.; Giapitzakis, I.; Scheffler, K.; Henning, A.: Evaluation of transmit efficiency and SAR for a tight fit transceiver human head phased array at 9.4 T. NMR in Biomedicine 30 (2), pp. 1 - 12 (2017)
Figures