3275
Adiabatic Null Passage for on resonance magnetization transfer preparation1Donders Institute for Brain, Cognition and Behaviour, Radboud University, Nijmegen, Netherlands, 2Erwin L. Hahn Institute for Magnetic Resonance Imaging, University Duisburg-Essen, Essen, Germany, 3Department of Radiology, Harvard Medical School, Boston, MA, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlsetown, MA, United States
Synopsis
Keywords: Magnetization transfer, RF Pulse Design & Fields, Adiabatic Null Passage
On-resonance bound pool saturation is the most efficient way of generating MTC. However, it suffers from excessive direct free water saturation due to RF pulse instabilities, and potential T2-weighting. We proposed a time-reversed adiabatic pulse (adiabatic null passage) for on-resonance MT preparation, by reversing the time-domain phase modulation function of a symmetric adiabatic pulse at its mid-point. We compared the MTC performance of ANP with binomial pulses using their MTR images. MTR values were reported through lines crossing CSF, showing high values at GM/WM and zero at CSF voxels. ANP improved the excessive saturation at regions with high B0 inhomogeneity.Introduction
Magnetization transfer (MT) contrast is generated by selectively saturating the bound pool (existing in the structure of macromolecules). By maintaining the bound pool in the saturation state during the course of imaging, the remaining magnetization of the free pool (that is not transferred) relaxes with a new relaxation rate (R1,sat) [1]. On-resonant saturation using binomial pulses is the most efficient way of generating MTC [2], however, is not commonly used because of the excessive direct saturation of free water caused by RF instabilities, which is exacerbated by B0 inhomogeneity [3]. In this study, we utilize the B0-insensitivity of the frequency-offset-corrected family of adiabatic pulses and their insensitivity to B1 amplitude above the adiabatic threshold to solve this problem. We propose a new class of adiabatic pulses creating a zero-flip angle for the free pool while saturating the bound pool, referred to as an adiabatic null passage (ANP) pulse. We expect that the ANP pulses improve MTC performance by i) reducing the excessive direct saturation compared to the conventional binomial pulse in areas with high B0 inhomogeneity, ii) reducing the sensitivity to small variations in B1 amplitude, iii) allowing the manipulation of the MT-effect without necessarily having to modify the pulse duration, and iv) minimizing the T2 weighting by allowing the use of short high-power pulses.Method
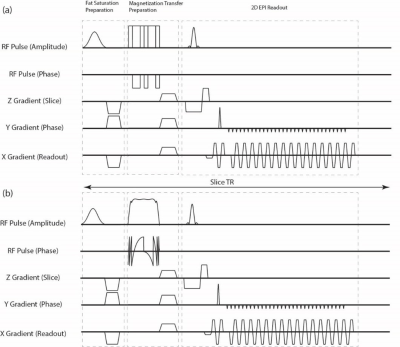
Theory: The movement of the bulk magnetization during the adiabatic pulse is governed by the effective field which is controlled by the amplitude and phase modulation functions. An adiabatic full passage uses symmetric AM/PM functions that make the effective field start from parallel with B0, crossing the origin at the mid-point, and ending anti-parallel with B0. In 1998, Norris [4] proposed that by simply reversing the phase of the pulse halfway through its time course, the effective field would land back again parallel with B0 resulting zero tip angle. In this study, we used this theory to tweak the TR-FOCI RF pulse, which has been shown to be SAR-efficient as well as robust to B0/B1+ inhomogeneities [5].Experiment: imaging was performed on a 3T scanner (Prisma, Siemens, Germany) with the 64-channel head coil. We compared the MT performance of our proposed ANP pulse (6 ms) with a phase-swapped 121-121 binomial scheme [3] (6 ms) with an MT preparation module followed by a simple 2D EPI readout. Figure 1 shows the diagram for both pulse sequences. The imaging parameters were: FOV = 240 x 240 mm2, TR = 1800 ms, TE = 11 ms, GRAPPA = 2, slice thickness = 3 mm, partial Fourier = 6/8. For each experiment, we acquired 40 volumes: The first 20 volumes without the MT pulse and the rest with the MT pulse. We chose the 20th volume as the reference image and the 40th as the MT-weighted image. We introduced τ, as the time between two successive MT pulses in an imaging volume. A field map was also acquired using a simple dual-echo GRE sequence with TEs of 2.2 ms and 4.6 ms with the same FOV, resolution, and slice position as the MTC volumes.
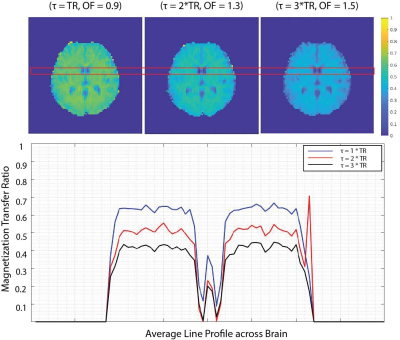
Analysis: The magnetization transfer ratio (MTR) image was calculated as 1 – (MT-weighted / reference). Before, the MTR calculation, the whole 40-volume scan was realigned based on the mid-volume using mcflirt in FSl for motion correction. The images were also corrected for bias field using SPM, and the brain was masked using BET. Given that CSF is a non-MT-active tissue with a long T2, we looked at the CSF signal as a reliable site to observe direct saturation. Therefore, we plotted an average of line profiles passing through CSF in the lateral ventricles, which gives us a comparison of MTR in GM, WM, and CSF. The field maps were generated using FSL.
Results
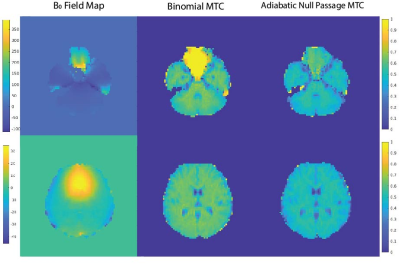
Figure 2 shows the MTR maps for a 6 ms ANP pulse in three different settings along with the average line MTR profiles. The ANP overdrive factor and parameters are chosen in the way that all three experiments are power matched so that the amount of free water direct saturation can be observed without any bias. The MTR values across the red line in figure 2 depict a high MTR at GM/WM with zero value for voxels containing CSF. Figure 3 compares the magnetization transfer performance of the binomial pulse with our proposed optimal adiabatic pulse. Excessive direct saturation is observed in the areas with high B0 inhomogeneity in the binomial MTC images, whereas in the ANP-MTC images is improved.Discussion
The proposed adiabatic null passage pulse could easily provide MT contrast. The high power of ANP could be deployed to apply the MT pulse less often ( τ = 2*TR, 3*TR) compared to binomial, which overcomes the issue of excessive direct saturation. The inherent frequency-offset correction of the TR-FOCI pulse results in a better MT performance in the areas with high B0 inhomogeneity (Figure 3). The immediate application of the proposed pulse could be time-of-flight magnetic resonance angiography [6] and arterial blood contrast fMRI [7] studies, especially in ultra-high fields.Acknowledgements
No acknowledgement found.References
1. Wolff, S.D. and R.S. Balaban, Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magnetic Resonance in Medicine, 1989. 10(1): p. 135-144.
2. Schneider, E., R.W. Prost, and G.H. Glover, Pulsed magnetization transfer versus continuous wave irradiation for tissue contrast enhancement. Journal of Magnetic Resonance Imaging, 1993. 3(2): p. 417-423.
3. Davies, N.P., I.R. Summers, and W. Vennart, Optimum setting of binomial pulses for magnetization transfer contrast. Journal of Magnetic Resonance Imaging, 2000. 11(5): p. 539-548.
4. Norris, D.G. 0° slice-selective RF pulses: MT-equivalence for multi-slice perfusion imaging. in Proceedings, ISMRM, 6th Annual Meeting, Sydney. 1998.
5. Hurley, A.C., et al., Tailored RF pulse for magnetization inversion at ultrahigh field. Magnetic resonance in medicine, 2010. 63(1): p. 51-58.
6. Schulz, J., R. Boyacioğlu, and D.G. Norris, Multiband multi slab 3 D time‐of‐flight magnetic resonance angiography for reduced acquisition time and improved sensitivity. Magnetic resonance in medicine, 2016. 75(4): p. 1662-1668.
7. Schulz, J., et al., Arterial blood contrast (ABC) enabled by magnetization transfer (MT): a novel MRI technique for enhancing the measurement of brain activation changes. bioRxiv, 2020.
Figures