3272
Extending inhomogeneous MT to assess both neuromelanin and myelin in brainstem structures1Radiology, Division of MR Research, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 2Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 3Department of Neurological Sciences, Rush University Medical Center, Rush Alzheimer's Disease Center, Chicago, IL, United States
Synopsis
Keywords: Magnetization transfer, Neuro, Brainstem
Application of neuromelanin MRI (NM-MRI) in the brainstem has been utilized in studies of neurological disorders such as Parkinson's disease. Acquisition of two types of MT preparation for inhomogeneous MT, MT applied at a single offset and then dual off-resonance frequencies, presents an advancement to NM-MRI by providing an acquisition with a myelin sensitive signal for complementary information. We demonstrate this in ex-vivo brainstem samples at 9.4 T, which allows comparison with other MRI microstructural techniques, as well as in-vivo at 3 T, where MT images provide a contrast comparable to NM-MRI by fast-spin-echo.Introduction
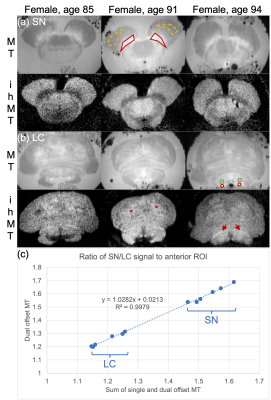
Use of neuromelanin (NM)-MRI in the brainstem has demonstrated changes associated with Parkinson’s and Alzheimer's diseases, and other neurological disorders, as well as alterations with aging1,2. NM-MRI is predominantly concerned with imaging melanin in dopaminergic neurons in two brainstem structures: the substantia nigra (SN) and the locus coeruleus (LC). Conditions in which NM-MRI has demonstrated utility are often associated with the presence of other pathologies, as well as age related effects. This includes white matter (WM) abnormalities, in the setting of demyelinating diseases or microangiopathies, that can degrade neural transmission within and outside the brainstem.Inhomogeneous Magnetization Transfer (ihMT) imaging provides a myelin sensitive and selectively specific signal based on validation with fluorescence microscopy in mice3. The ihMT experiment includes acquisitions with MT preparations applied at a single offset frequency, and separately at dual offset frequencies, i.e. positive and negative versions of the same value4. NM-MRI often involves averaging to resolve the relatively small LC. Since the LC’s contrast is associated with a lower fractional size of the macromolecular pool5, the LC signal following single and dual frequency MT preparations should be relatively similar. Thus, images prepared with single and dual frequency MT pulses might be utilized with the sum for MT NM-MRI, and the difference for ihMT. We present novel application of the ihMT experiment for NM-MRI, and to provide a myelin sensitive signal.
Methods
MRI of formalin-fixed brainstem samples in PBS from three female decedents (ages 88-94 years) was conducted with an 83-mm quadrature volume coil on a 9.4T scanner (Biospec 94/20, Bruker). Data for NM-MRI and ihMT were acquired with modifications to a 3D MPRAGE style sequence6, with a preparation of four 3-ms MT pulses applied every 100 ms for 1 s. Dual frequency MT preparations were achieved by cosine modulation. Multi-slice, multi-echo and 3D spin-echo EPI sequences were also applied for T2 mapping and DTI respectively. An air heater system with fiber optic temperature probe (SA Instruments) was used to heat the sample and maintain it at a physiological 37 °C during MRI.The potential for clinical translation was demonstrated in-vivo with MRI of three healthy volunteers (2 males, 1 female, ages 22-39 years), again with a 3D MPRAGE style sequence6, on a 3T scanner (Signa Premier, GE Healthcare) using a 21-channel head and neck coil. The MT preparation consisted of 5-ms MT pulses every 100 ms for 1 s. A multi-slice fast-spin-echo (FSE) sequence was also acquired for NM-MRI.
Results
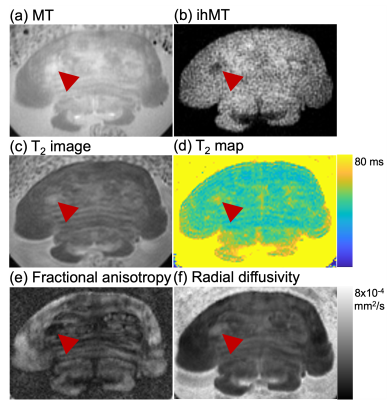
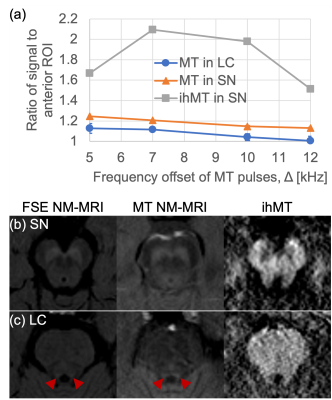
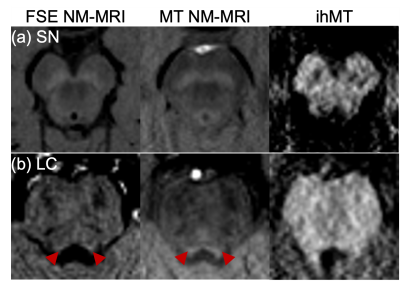
As in prior NM-MRI literature, ex-vivo the SN and LC appeared hyperintense for MT, and corresponding ihMT images showed increased signal in WM structures such as the corticospinal tract and medial lemniscus (asterisks and arrows in Figure 1 respectively). Use of contrast from the sum of single and dual frequency MT images correlated with the contrast from dual offset MT (Figure 1c). In one case, at the LC level an anomalous hypointense signal (indicative of myelin loss) was also observed in the pons from the ihMT images (arrowheads in Figure 2). It corresponded with a region of long T2, low fractional anisotropy (FA), and high radial diffusivity (RD), but appears most prominent relative to surrounding signal in the pons with ihMT.In-vivo, contrast from the SN and LC for MT NM-MRI as a function of RF offset at which the MT pulses were applied in the ihMT experiment peaked at Δ = 5 kHz, but at Δ = 10 kHz for ihMT in the SN (Figure 3a). The MT images show comparable contrasts with those from the FSE acquisition, particularly for the SN (Figures 3-5). As in the ex-vivo images, the ihMT image shows a hypo-intensity from the region corresponding with the SN, and a relative lack of contrast in the pons at the level of the LC.
Discussion
We demonstrated how the ihMT experiment can provide both MT and ihMT images for assessment of NM and myelin in the brainstem, which separately have shown utility in studies of various neurological disorders1,2,7. Ex-vivo samples allowed comparison between ihMT data with microstructural information provided by other contrast mechanisms, as highlighted in one case of potential myelin loss (Figure 2). The greatest contrast for NM-MRI was at Δ = 5 kHz (Figure 3a), which is not maximal for ihMT as expected from prior literature4. Thus, ihMT for NM-MRI might be further optimized, including use of an FSE readout for greater signal and scan time reduction8.Conclusion
Our demonstration of successful NM-MRI using the ihMT experiment represents a novel application of the technique. Further work in a larger sample size will be needed to demonstrate the added benefit of both NM and myelin indices from the same brainstem structures.Acknowledgements
We gratefully acknowledge support from Rush Alzheimer's Disease Research Center (P30AG072975) and the National Institute on Aging of the National Institutes of Health under award number R01AG071638.References
1. Mitchell, T. et al. Emerging Neuroimaging Biomarkers Across Disease Stage in Parkinson Disease: A Review. JAMA Neurology 78, 1262–1272 (2021).
2. Beardmore, R., Hou, R., Darekar, A., Holmes, C. & Boche, D. The Locus Coeruleus in Aging and Alzheimer’s Disease: A Postmortem and Brain Imaging Review. JAD 83, 5–22 (2021).
3. Duhamel, G. et al. Validating the sensitivity of inhomogeneous magnetization transfer (ihMT) MRI to myelin with fluorescence microscopy. NeuroImage 199, 289–303 (2019).
4. Varma, G., Duhamel, G., de Bazelaire, C. & Alsop, D. C. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin: Magnetization Transfer from Inhomogeneously Broadened Lines. Magn. Reson. Med. 73, 614–622 (2015).
5. Priovoulos, N. et al. Unraveling the contributions to the neuromelanin-MRI contrast. Brain Struct Funct 225, 2757–2774 (2020).
6. Varma, G. et al. Three-dimensional inhomogeneous magnetization transfer with rapid gradient-echo (3D ihMTRAGE) imaging. Magnetic Resonance in Medicine 84, 2964–2980 (2020).
7. Alsop, D. C. et al. Inhomogeneous magnetization transfer imaging: Concepts and directions for further development. NMR in Biomedicine, e4808.
8. Taso, M. et al. Fast-spin-echo versus rapid gradient-echo for 3D magnetization-prepared acquisitions: Application to inhomogeneous magnetization transfer. Magnetic Resonance in Medicine.
Figures