3269
Amide Proton Transfer Weighted Combined with Diffusion Kurtosis Imaging for Predicting Lymph Node Metastasis in Cervical Cancer
Yue Wang1, Shifeng Tian1, and Ailian Liu1
1the First Affiliated Hospital of Dalian Medical University, Dalian, China
1the First Affiliated Hospital of Dalian Medical University, Dalian, China
Synopsis
Keywords: Pelvis, Cancer
Cervical cancer (CC) is one of the most common gynecological malignancies worldwide, and its incidence is gradually increasing. Acurate identification of lymph node metastases (LNM) is crucial for predicting prognosis and choosing the best available treatment. Amide proton transfer weighted (APTw) imaging can detect the chemical exchange rate of between water and endogenous mobile proteins, peptides or polypeptides[1]. Diffusion kurtosis imaging (DKI) can reflect the limited diffusion movement of water molecules in tissue and the complexity of tissue microstructure [2]. This study is aimed to investigate the quantitative prediction of LNM in CC by APTw combined with DKI.Introduction
Cervical cancer (CC) is one of the most common gynecological malignancies worldwide, and its incidence is gradually increasing. Acurate identification of lymph node metastases (LNM) is crucial for predicting prognosis and choosing the best available treatment. Amide proton transfer weighted (APTw) imaging can detect the chemical exchange rate of between water and endogenous mobile proteins, peptides or polypeptides[1]. Diffusion kurtosis imaging (DKI) can reflect the limited diffusion movement of water molecules in tissue and the complexity of tissue microstructure [2]. This study is aimed to investigate the quantitative prediction of LNM in CC by APTw combined with DKI.Methods
Data of 17 LNM(+) and 50 LNM(-) patients with CC were retrospectively analyzed. 3.0T MRI scan was performed before the operation. The scanning sequence included APTw and DKI(Tab 1). After post-processing, quantitative APT, mean kurtosis (MK), and mean diffusivity (MD) maps were obtained (Fig 1). The APT, MK, and MD values of the two groups of cases were measured by two observers, and intra-class correlation coefficients (ICC) were used to test the consistency of the measurement results. The independent samples t-test or Mann-Whitney U test was used to compare the differences in the values of each parameter between the two groups.Results
The two observers measured the data in good agreement (ICC>0.75). The values of APT, MK, and MD in the LNM(+) group were 3.277±0.498 %, 0.517±0.209, and 1.145±0.426 μm2/ms, respectively; in the LNM(-) group, were 2.698±0.597 %, 0.401±0.148 and 1.258±0.567 μm2/ms, respectively. The APT and MK values of the LNM(+) group were higher than those of the LNM(-) group (P<0.05), while there was no significant difference in the MD values (P>0.05). APT value, MK value, and APT+MK value obtained an AUC of 0.777, 0.691, and 0.812, respectively, when predicting LNM status of CC(Fig 2, Tab 2-3).Discussion:
Our results show that APT value of CC with LNM(+) is higher than that of CC with LNM(-), because the rapid cell proliferation and relatively insufficient blood supply in CC with LNM may make tumor tissue more susceptible to microscopic necrosis, and more mobile proteins and peptides will be released into the surrounding environment. All these factors increase the local protein and peptide concentrations in CC tumors, resulting in an increase in APT value. The MK value of LNM(+)CC is higher than that of LNM(-)[3]. We believe that the internal components of LNM(+)CC are more diverse and the neovascularization is more abundant, which increases the complexity of the microenvironment structure and the deviation caused by the diffusion movement of water molecules, leading to the higher MK value than that of LNM(-). In this study, there was no statistically significant difference between the MD values of the two groups of cases, presumably due to the similar extracellular space of the tumor and the diffusion resistance of water molecules between the two groups[4].Conclusion:
APTw and DKI can quantitatively predict LNM status of CC and thus have great potential clinical diagnostic values.Acknowledgements
None.References
[1] Lin S, Gao K, Gu S, et al. Worldwide trends in cervical cancer incidence and mortality, with predictions for the next 15 years. Cancer, 2021, 127(21):4030-4039.
[2] Sun PZ. Consistent depiction of the acidic ischemic lesion with APT MRI-Dual RF power evaluation of pH-sensitive image in acute stroke. Magn Reson Med, 2022, 87(2):850-858.
[3] Li A, Yuan G, Hu Y, et al. Renal functional and interstitial fibrotic assessment with non-Gaussian diffusion kurtosis imaging. Insights Imaging, 2022, 13(1):70.
[4] Zhang D, Geng X, Suo S, et al. The predictive value of DKI in breast cancer: Does tumour subtype affect pathological response evaluations? Magn Reson Imaging, 2022, 85:28-34.
Figures

Figure 1. A 30-year-old female patient with poorly differentiated squamous cell carcinoma of the cervix with metastasis to the right obturator lymph node.(A) T2WI showing slightly high signal in the cervical cancer lesions (red arrow). (B) The fusion image of APT pseudo-color image and T2WI; the APT value is 3.350 %. (C and D)The MK (C) and MD (D) pseudo-color images of DKI sequence; their quantitative values are 0.424, 0.753 μm2/ms, respectively.
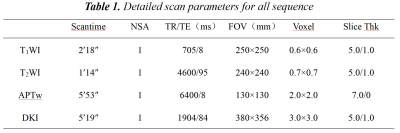
Table 1. Detailed scan parameters for all sequence
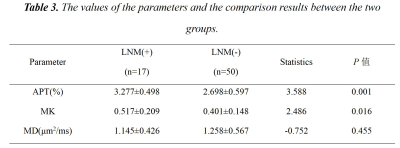
Table 2. The values of the parameters and the comparison results between the two groups.
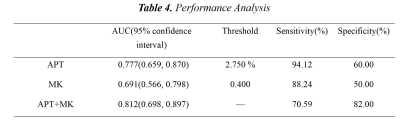
Table 3. Performance Analysis
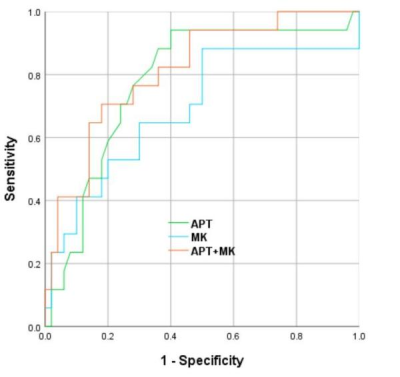
Figure 2. The ROC curves of amide proton transfer (APT), mean kurtosis (MK), and APT+MK values for predicting the LNM of cervicalcarcinoma.
DOI: https://doi.org/10.58530/2023/3269