3261
Application of diffusion imaging and dynamic contrast-enhanced MRI in evaluating the proliferation status of endometrial carcinoma1Department of MR, the First Affiliated Hospital, Xinxiang Medical University, Weihui, China, 2MR Research China, GE Healthcare, Beijing, China
Synopsis
Keywords: Pelvis, Cancer
Intravoxel incoherent motion (IVIM) is able to reflect the true diffusion, microcirculatory perfusion, average diffusion, and structural complexity in tissues. Dynamic contrast-enhanced MRI (DCE-MRI) can be used to analyze the dynamic distribution of contrast agents through the pharmacokinetic model to quantitatively detect the blood supply of biological tissues. Our results showed that IVIM and DCE-MRI-derived parameters such as D, α, Ktrans, and Kep were associated with Ki-67 status in EC, and the combination of D and Kep may serve as a superior imaging marker for the identification of low-proliferation and high-proliferation EC.Introduction
Intravoxel incoherent motion (IVIM) is able to reflect the true diffusion, microcirculatory perfusion, average diffusion, and structural complexity in tissues. Dynamic contrast-enhanced MRI (DCE-MRI) can be used to analyze the dynamic distribution of contrast agents through the pharmacokinetic model to quantitatively detect the blood supply of biological tissues. Our results showed that IVIM and DCE-MRI-derived parameters such as D, α, Ktrans, and Kep were associated with Ki-67 status in EC, and the combination of D and Kep may serve as a superior imaging marker for the identification of low-proliferation and high-proliferation EC.Material and Methods
A total of 70 patients were enrolled in this study. A 1.5 T MR system (Optima MR360, GE Healthcare, Waukesha, WI, USA) with a 12-channel phased-array body coil were used for scanning. The monoexponential model was performed by using two b values (0, 1000 s/mm2). The biexponential and stretched models were performed by using ten b values (b = 0, 20, 40, 80, 160, 200, 400, 600, 800, and 1000 s/mm2). The DCE-MRI images were obtained using a three-dimensional liver acquisition with volume acceleration (3D-LAVA) sequence with a temporal resolution of 9 s. Sequential images were acquired from 9 s before i.v. injection of gadopentetate dimeglumine (Gd-DTPA, Bayer Pharmaceutical, Berlin, Germany; 0.2 mL/kg, 3.0 mL/s) to 360 s after. The ROIs, excluding areas with large vessels, hemorrhagic, calcified, cystic, and necrotic, were drawn along the edge at the maximum cross-section of the tumor.MedCalc 15.0 and SPSS 23.0 were employed for statistical analyses. The independent sample t-test and Mann-Whitney U test were applied for between-group comparison. The receiver operating characteristic (ROC) curve and Delong test were performed to evaluate and compare the diagnostic performance of each parameter. The logistic regression analyses were used to derive a prediction model. The correlation between each parameter and Ki-67 was described by Spearman rank correlation. Results with P < 0.05 were considered to be significant. The multi-variates logistic regression and bootstrap (1000 samples) were used to establish and evaluate the optimal model for Ki-67 status prediction, respectively.Results
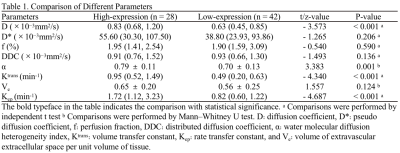
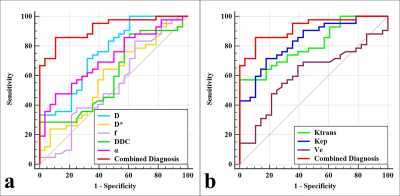
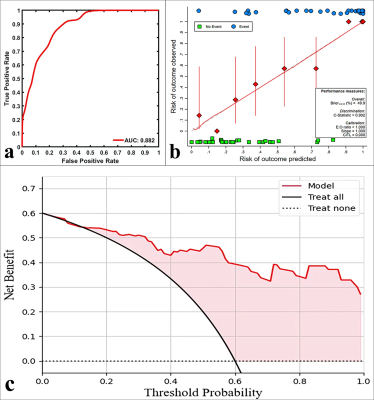
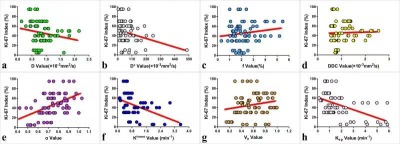
As shown in Table 1, the D, Ktrans, and Kep were lower, and α was higher in the high-proliferation group than in the low-proliferation group (all P < 0.05). D and Kep were independent predictors for Ki-67 status in EC, and the combination of these parameters had an optimal diagnostic efficacy (AUC, 0.920; sensitivity, 85.71 %; specificity, 89.29 %), which was significantly better than D (AUC = 0.753, Z = 2.874, P = 0.004), α (AUC = 0.715 , Z = 3.505, P = 0.001), Ktrans (AUC = 0.808 , Z = 2.741, P = 0.006), and Kep (AUC = 0.832, Z = 2.147, P = 0.032)(Figure 1) .The validation model showed good accuracy (AUC, 0.882; 95% CI, 0.861 - 0.897) and consistency (C-statistic, 0.902) (Figure 2). D, Kep, Ktrans , and α were mildly negatively, moderately negatively, mildly negatively, and mildly positively correlated with Ki-67 index, respectively (r = -0.271, -0.534, -0.409, and 0.488, all P < 0.05) (Figure 3).Discussion
Compared with low-proliferation EC, high-proliferation EC have an increase in cellular density, nuclear atypia, and microscopic necrosis, which not only can limit the diffusion velocity of water molecules and intravoxel diffusion heterogeneity, also decrease capillary microcirculation perfusion, and intravoxel diffusion heterogeneity, leading to changes in signal intensity (SI) of IVIM and DCE-MRI (5, 6). In the present study, both Ktrans and Kep values were significantly greater in the high-proliferation group than in the low-proliferation group. We speculate that it may be due to the high proliferation and metabolism of EC cells in the high-proliferation group compared with the low proliferation group, which results in abundant neovascularization and immature vessel walls, accelerating the rate of intra- and extravascular contrast exchange and eventually causing an increase in Ktrans and Kep values. The D value was significantly lower in the high-proliferation group than in the low-proliferation group and was one of the independent predictors for the discrimination between the two, which is consistent with the above findings and suggests that the D value has a positive significance in the prediction of Ki-67 status in EC. In addition, α is a parameter generated by stretched exponential model IVIM to reflect the complexity of the tissue. Established investigations have found that due to the higher level of intravoxel microscopic necrotic foci, heterogeneous cellularity, and heterogeneous cellularity, the tissue complexity of high-proliferation lesions tends to increase compared with that of low-proliferation lesions, and therefore the α value tends to decrease.Conclusion
IVIM and DCE-MRI-derived parameters such as D, α, Ktrans, and Kep were associated with Ki-67 status in EC, and the combination of D and Kep may serve as a superior imaging marker for the identification of low-proliferation and high-proliferation EC.Acknowledgements
The Roentgen Imaging Research Project (HN-20201017-002), the Key Project of Henan Province Medical Science and Technology Project (LHGJ20200519, 2018020367).References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33.
2. Ocak B, Atalay FÖ, Sahin AB, et al. The impact of Ki-67 index, squamous differentiation, and several clinicopathologic parameters on the recurrence of low and intermediate-risk endometrial cancer. Bosn J Basic Med Sci. 2021;21:549-554.
3. Iima M. Perfusion-driven Intravoxel Incoherent Motion (IVIM) MRI in Oncology: Applications, Challenges, and Future Trends. Magn Reson Med Sci. 2021;20:125-138.
4. Kang SR, Kim HW, Kim HS. Evaluating the Relationship Between Dynamic Contrast-Enhanced MRI (DCE-MRI) Parameters and Pathological Characteristics in Breast Cancer. J Magn Reson Imaging. 2020;52:1360-1373.
5. Meyer HJ, Höhn AK, Surov A. Associations between dynamic-contrast enhanced MRI and tumor infiltrating lymphocytes and tumor-stroma ratio in head and neck squamous cell cancer. Cancer Imaging. 2021;21:60.
6. Huang Z, Li X, Wang Z, et al. Application of Simultaneous 18 F-FDG PET With Monoexponential, Biexponential, and Stretched Exponential Model-Based Diffusion-Weighted MR Imaging in Assessing the Proliferation Status of Lung Adenocarcinoma. J Magn Reson Imaging. 2021;10.1002/jmri.28010.
Figures