3253
Diagnostic ability of APT in differentiating ovarian high-grade serous carcinomas from borderline serous tumors
Jiacheng Song1, Aining Zhang1, Xiance Zhao2, Yishi Wang3, Zhiwei Shen3, and Ting Chen1
1the first affiliated hospital of Nanjing Medical University, Nanjing, China, 2Philips Healthcare, Shanghai, China, 3Philips Healthcare, Beijing, China
1the first affiliated hospital of Nanjing Medical University, Nanjing, China, 2Philips Healthcare, Shanghai, China, 3Philips Healthcare, Beijing, China
Synopsis
Keywords: Urogenital, CEST & MT
The precise assessment of ovarian tumor, which is complex in presentation and classification, is important for clinical decision making. The value of amide proton transfer weighted (APTw) imaging in the assessment of ovarian lesion has not been comprehensively researched. In this study, we investigated the value of APTw imaging in differentiating ovarian high-grade serous carcinomas from borderline serous tumors. It was found that APT value of high-grade tumor was significantly lower than that of borderline tumor. Our finding indicates the potential of using APTw imaging as a novel method for the differentiation diagnosis of ovarian cancers.Introduction
Ovarian tumors usually present with cystic-solid components and have various subtypes. The differentiation of high-grade serous carcinomas from borderline serous tumors is crucial for clinical surgery practice. However, the accuracy of intraoperative frozen-section, which ranges from 45% to 87%[1], is not always sufficient for clinical decision making during the surgery. Therefore, more precise preoperative assessment of the tumor is needed for an early identification of the tumor grade. APT is a non-invasive method which can display the signal intensity of amide protons contained in proteins and peptides. It has been mainly researched on brain, breast, and rectum. The correlation between APT and tumor grade, Ki-67 index, and pseudo progression were found in previous studies [2][3][4]. Potentially due to the complexity in ovarian tumor classification and the artifacts from ovarian tumor cystic components on APTw image, the characterization of ovarian cancer under APTw imaging has not been comprehensively studied. Therefore, this study intends to evaluate the value of APTw imaging in differentiating ovarian tumors high-grade serous carcinomas from borderline serous tumors.Materials and Methods
This study was approved by ethical committee of First Affiliated Hospital of Nanjing Medical University. Written informed consent was obtained before MR. 30 patients with ovarian high-grade serous carcinomas and 8 patients with ovarian borderline serous tumors were recruited from November 2021 to October 2022. MRI was performed on a 3T MR system (Ingenia CX, Philips Healthcare, Best, Netherland) with a 16-channel abdomen coil. APTw images were acquired with a 3-dimensional (3D) turbo-spin-echo sequence (continuous radiofrequency excitation pulses with an amplitude of 2μT and duration of 2s were used for saturation, TR = 6491 ms, TE = 8.3ms, turbo factor = 174, slice thickness = 6 mm, SENSE factor =1.6, FOV = 230 × 180 × 60 mm3, and acquisition resolution = 1.8 × 1.8 mm2). The spectral pre-saturation inversion-recovery (SPIR) method is used for fat suppression. APTw images were automatically generated via the Philips post-processing workstation. The drawing of the region of interest (ROI) is shown in figure1. For each lesion, five ROIs were drawn and the average of measured values were calculated. To compare the mean values of the two groups, independent sample t-test was used. P<0.05 was considered as significant.Results
30 patients with ovarian high-grade serous carcinomas and 8 patients with ovarian borderline serous tumors were included in the analysis. The basic patients’ characteristics are shown in table 1. Patients with high-grade serous carcinomas were older than borderline serous tumors (P<0.05). Tumor size and accompanied ascites didn’t show statistical difference between the two groups. The example images of high grade serous carcinomas and borderline serous tumor were shown in Figure 2. APT value of borderline serous tumors was significantly higher than that of high-grade serous carcinomas (P<0.05).Discussion
To the best of our knowledge, this is the first study to show the potential of APT value in differentiating ovarian high-grade serous carcinomas from borderline serous tumors. There is one former research focused on APT in ovarian lesions, in which higher APT value was reported in mucinous cystadenoma when comparing to serous cystadenoma. Because APT value can be easily influenced by gas, fat of fluid in the abdomen, it is not straight forward to use this technique to study organs in the abdomen, such as ovarian. Ovarian lesions are also complicated and varied so that the classification of the lesion is challenging, which hinder the decision-making for precise treatment. Therefore, it is relevant to develop new method for accurate differentiation of high-grade serous carcinomas from borderline serous tumors. In our results, APT value in borderline serous tumors were significantly higher than that of high-grade serous carcinomas. This may be due to the larger cytoplasmic ratio of borderline serous tumors[5], which allows more protein to be exchanged in the microenvironment. Another reason may be the low pH values in high-grade serous carcinomas resulted from hypoxia[6]. Low pH value will further reduce APT valve of high-grade serous carcinomas.Conclusion
By using APTw imaging, ovarian high-grade serous carcinomas can be differentiated from borderline serous tumors. APTw imaging is potentially a novel method for the differentiation diagnosis of ovarian carcinoma.Acknowledgements
No acknowledgement found.References
1. Li Y, Jian J, Pickhardt PJ, et al. (2020) MRI-Based Machine Learning for Differentiating Borderline From Malignant Epithelial Ovarian Tumors: A Multicenter Study. J Magn Reson Imaging 52:897–904. doi: 10.1002/jmri.27084 2. Zhang J, Zhu W, Tain R, et al. (2018) Improved Differentiation of Low-Grade and High-Grade Gliomas and Detection of Tumor Proliferation Using APT Contrast Fitted from Z-Spectrum. Mol Imaging Biol 20:623–631. doi: 10.1007/s11307-017-1154-y 3. Nishie A, Takayama Y, Asayama Y, et al. (2018) Amide proton transfer imaging can predict tumor grade in rectal cancer. Magn Reson Imaging 51:96–103. doi: 10.1016/j.mri.2018.04.017 4. Bai Y, Lin Y, Zhang W, et al. (2017) Noninvasive amide proton transfer magnetic resonance imaging in evaluating the grading and cellularity of gliomas. Oncotarget 8:5834–5842. doi: 10.18632/oncotarget.13970 5. Weir MM, Bell DA (2001) Cytologic identification of serous neoplasms in peritoneal fluids. Cancer 93:309–318. doi: 10.1002/cncr.9045 6. Todeschini P, Salviato E, Romani C, et al. (2021) Comprehensive profiling of hypoxia-related mirnas identifies mir-23a-3p overexpression as a marker of platinum resistance and poor prognosis in high-grade serous ovarian cancer. Cancers (Basel). doi: 10.3390/cancers13133358Figures
Table1: The basic patients' characteristics
HGSC: high-grade serous carcinomas; BST: borderline serous tumors
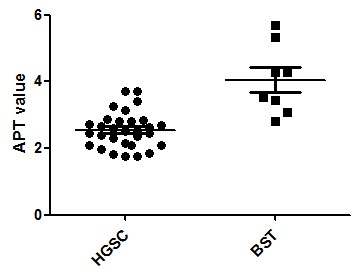
Figure 1. APT value
comparison between high-grade serous carcinomas and borderline serous tumors
HGSC: high-grade
serous carcinomas; BST: borderline serous tumors
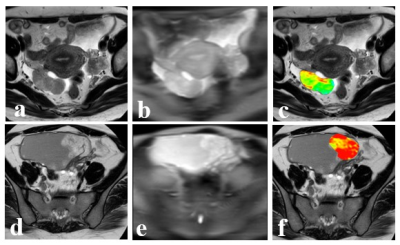
Figure 2. 48
years old patient with right ovary high-grade serous carcinoma. a: axial T2WI
show right ovary solid lesion with long length of 4.5cm; b: B0 image; c: T2WI
overlaid with pseudo color APT image (APT value 2.45%). 25 years old patient
with right ovary borderline serous tumor. d: axial T2WI show right
ovary cystic-solid lesion with solid part long length of 4cm; e: B0 image; f: T2WI
overlaid with pseudo color APT image (APT value 4.27%).
DOI: https://doi.org/10.58530/2023/3253