3245
Harmonization of the multisite ALPS-index based on DTI-ALPS using the combined association test1Department of Radiology, Juntendo University Graduate School of Medicine, Tokyo, Japan, 2Department of Innovative Biomedical Visualization (iBMV), Nagoya University Graduate School of Medicine, Aichi, Japan, 3Department of Radiological Sciences, Graduate School of Human Health Sciences, Tokyo Metropolitan University, Tokyo, Japan, 4Department of Radiology, University of Tokyo, Tokyo, Japan, 5Department of Radiology, Toho University Omori Medical Center, Tokyo, Japan, 6Department of Radiology, Nagoya University Graduate School of Medicine, Aichi, Japan
Synopsis
Keywords: Data Processing, Data Processing, Diffusion MRI, ALPS-index, DTI-ALPS, Harmonization, multisite study
Diffusion tensor image analysis along the perivascular space (DTI-ALPS) is a promising noninvasive method for indirectly evaluating the glymphatic system. However, the ALPS-index calculated from diffusion MRI data collected at multiple sites should be harmonized to avoid site-related effects. We applied the combined association test (ComBat), which uses regression of covariates with empirical Bayes, for harmonizing the ALPS-index. ComBat mitigated site-related effects, increased statistical power to differentiate Alzheimer’s disease and cognitive normal, and improved the correlation between the ALPS-index and cognitive function. Thus, ComBat harmonization can be applied to evaluate the glymphatic system using the ALPS-index in large multisite studies.
INTRODUCTION:
According to the glymphatic system hypothesis1, there is increasing interest in interstitial fluid movement in the brain associated with the excretion of waste products1-5. Diffusion tensor image analysis along the perivascular space (DTI-ALPS) is a promising noninvasive method for indirectly evaluating the glymphatic system6. However, the ALPS-index is influenced by site-related effects, such as scanner and protocol differences4. Therefore, a harmonization technique is required in multisite ALPS-index research to increase statistical power, which will help detect subtle pathological changes with high repressibility and reliability.In this study, the combined association test (ComBat)7, which uses regression of covariates with empirical Bayes, was applied for harmonizing the ALPS-index to reduce site-related effects. Moreover, we confirmed the relation between the ALPS-index and cognitive function.
METHODS:
Study participantsWe obtained the diffusion MRI (dMRI) data of 45 Alzheimer’s disease (AD) patients and 82 cognitive normal (CN) participants from the Alzheimer's Disease Neuroimaging Initiative study (https://adni.loni.usc.edu/) involving three 3T-MRI scanners (Siemens Healthcare Prisma fit, GE Healthcare Signa HDxt, and Discovery MR750; Table 1). To reduce sampling bias and focus on measurement bias, the participants were matched across scanners for age, sex, education, ethnicity, and handedness. The dMRI acquisition parameters are shown in Table 2.
Diffusion MRI preprocessing
All dMRI data were corrected for susceptibility, eddy current-induced geometric distortions8, and intervolume subject motion9. The ALPS-index was calculated after diffusion tensor fitting using preprocessed dMRI data3, 5, 10. The positions of regions of interest (ROIs) were visually checked for each participant, and manual corrections were not performed because all ROIs were correctly placed.
Harmonization of the ALPS-index using ComBat
ComBat7 was applied to harmonize the ALPS-index, with age, sex, and subject type (i.e., AD or CN) included as biological covariates.
Statistical analysis
To evaluate harmonization performance, Welch’s t-test was performed to compare the difference in the ALPS-index between AD and CN participants before and after harmonization. Moreover, to confirm the relation between the ALPS-index and cognitive function, Pearson’s correlation coefficient was calculated.
RESULTS:
Figure 1 shows the ALPS-index distribution of each scanner for CN before and after harmonization. Before ComBat harmonization, the ALPS-index was the largest for Prisma fit, followed by Discovery MR750 and Signa HDxt. The differences in the left and right ALPS-index among scanners were 0.14 and 0.20, respectively. After harmonization, the ALPS-index distribution was closer among scanners, with a difference up to 0.02. Consequently, the group difference between AD and CN increased by around half (Figure 2), and it was significant after harmonization. Furthermore, Pearson’s correlation coefficient between the ALPS-index and cognitive score was high after harmonization (Table 3).DISCUSSION:
This study applied ComBat to harmonize the ALPS-index. ComBat successfully mitigated site-related effects and differentiated the ALPS-index between AD and CN. Furthermore, the harmonized ALPS-index was strongly correlated with cognitive function.Taoka et al.4 reported how the ALPS-index is influenced by MRI acquisition parameters. First, the ALPS-index changed by around 0.10 in the scan-rescan dMRI. Our results showed that ComBat harmonized the multisite ALPS-index and reduced variance among scanners. Second, the ALPS-index decreased as echo time increased and the number of MPG axes decreased. Our results showed that the ALPS-index was higher for Prisma fit (shorter echo time and more MPG direction) than for the other scanners. This tendency of ALPS-index change depending on dMRI acquisition parameters is consistent with previous findings4.
ComBat harmonization removed the scanner difference from multiscanner data and reduced the ALPS-index distribution. Consequently, the group difference after harmonization was approximately 1.5 times the original value, and the P-value decreased. Regarding the detection of a significant difference (P < .05) in the ALPS-index with up to 0.80 statistical power, while the sample size needs to be at least 454 (151 AD and 303 CN subjects) before harmonization, it needs to be at least 188 (63 AD and 125 CN subjects) after harmonization. Thus, ComBat harmonization could reduce the cost for data correction in a multisite study to detect changes in the glymphatic system associated with pathology, using the ALPS-index.
It has been reported that the ALPS-index reflects glymphatic system function and cognition in AD2. Although cognitive scores were not included in the ComBat model, our results showed that ComBat harmonization improved the correlation between the ALPS-index and cognitive function by reducing the variance caused by scanner difference, which is consistent with previous findings2. Thus, ComBat could clarify the relation between the ALPS-index and cognitive function in a multisite study.
To evaluate the function of the glymphatic system, the major approach is a tracer study1, 11, 12. However, it is invasive and causes pain. On the contrary, DTI-ALPS using MRI is a noninvasive technique. Therefore, the ALPS-index based on DTI-ALPS is promising to evaluate the glymphatic system noninvasively in a multisite study with a large sample size, and ComBat harmonization has the potential to enable its use.
CONCLUSION:
ComBat harmonization was useful for mitigating site-related effects associated with the ALPS-index based on DTI-ALPS, and it retained and clarified the relation between the ALPS-index and cognitive function. Thus, in a multisite study with a large cohort, changes in the glymphatic system caused by pathological changes could be detected with high reliability after harmonizing the ALPS-index.Acknowledgements
Data collection and sharing for this project were funded by the ADNI (National Institutes of Health grant no. U01 AG024904) and Department of Defense ADNI (Department of Defense award number W81XWH-12-2-0012). This study was partially supported by the Juntendo Research Branding Project, JSPS KAKENHI (grant nos. JP16H06280, JP18H02772, 19K17244, 21K07690), a Grant-in-Aid for Special Research in Subsidies for ordinary expenses of private schools from The Promotion and Mutual Aid Corporation for Private Schools of Japan, the Brain/MINDS Beyond program (grant no. JP19dm0307101) of the Japan Agency for Medical Research and Development (AMED), and AMED under grant number JP21wm0425006. The Department of Innovative Biomedical Visualization (iBMV), Nagoya University Graduate School of Medicine, is financially supported by Canon Medical Systems Corporation.References
1. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012;4:147ra111.
2. Kamagata K, Andica C, Takabayashi K, et al. Association of MRI Indices of Glymphatic System With Amyloid Deposition and Cognition in Mild Cognitive Impairment and Alzheimer Disease. Neurology 2022.
3. Ma X, Li S, Li C, et al. Diffusion Tensor Imaging Along the Perivascular Space Index in Different Stages of Parkinson's Disease. Front Aging Neurosci 2021;13:773951.
4. Taoka T, Ito R, Nakamichi R, et al. Reproducibility of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating interstitial fluid diffusivity and glymphatic function: CHanges in Alps index on Multiple conditiON acquIsition eXperiment (CHAMONIX) study. Jpn J Radiol 2022;40:147-158.
5. Yang G, Deng N, Liu Y, Gu Y, Yao X. Evaluation of Glymphatic System Using Diffusion MR Technique in T2DM Cases. Front Hum Neurosci 2020;14:300.
6. Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol 2017;35:172-178.
7. Fortin JP, Parker D, Tunç B, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage 2017;161:149-170.
8. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003;20:870-888.
9. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016;125:1063-1078.
10. Taoka T, Fukusumi A, Miyasaka T, et al. Structure of the Medullary Veins of the Cerebral Hemisphere and Related Disorders. Radiographics 2017;37:281-297.
11. Gaberel T, Gakuba C, Goulay R, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke 2014;45:3092-3096.
12. Iliff JJ, Lee H, Yu M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 2013;123:1299-1309.
Figures
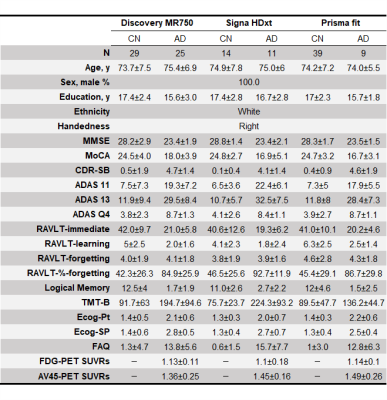
Table 1. Demographic characteristics of the study participants.
Abbreviations: MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; CDR-SB, Clinical Dementia Rating Sum of Boxes; ADAS, Alzheimer's Disease Assessment Scale; TMT-B, Trail Making Test Part B; ECog, Everyday Cognition; FAQ, Functional Activities Questionnaire; FDG, 18F-fluorodeoxyglucose; AV45, 18F-florbetapir.
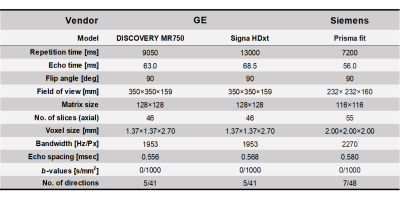
Each dMRI examination was performed using three 3T-MRI scanners (Siemens Prisma fit, GE signa HDxt, and Discovery MR750). All dMRI data were corrected for susceptibility, eddy current-induced geometric distortions, and intervolume subject motion. The ALPS-index was calculated according to previous studies after diffusion tensor fitting using preprocessed dMRI data.
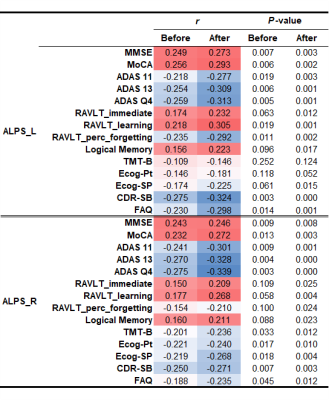
Table 3. The correlation between the ALPS-index and cognitive score.
ComBat harmonization improved the correlation between the ALPS-index and cognitive function by reducing the variance caused by scanner difference.
Abbreviations: MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; CDR-SB, Clinical Dementia Rating Sum of Boxes; ADAS, Alzheimer's Disease Assessment Scale; TMT-B, Trail Making Test Part B; ECog, Everyday Cognition; FAQ, Functional Activities Questionnaire.
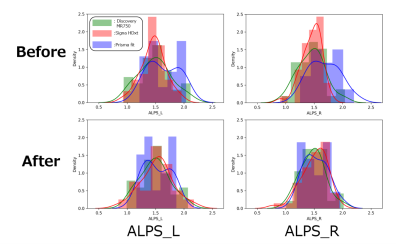
Figure 1. ALPS-index distribution in CN participants before and after harmonization.
The ALPS-index distribution was closer after harmonization. The solid curve indicates kernel density estimation. Green, Discovery MR750; red, Signa HDxt; blue, Prisma fit. Before ComBat harmonization, the ALPS-index was the largest for Prisma fit, followed by Discovery MR750 and Signa HDxt. The differences in the left and right ALPS-index among scanners were 0.14 and 0.20, respectively. After harmonization, the ALPS-index distribution was closer among scanners, with a difference up to 0.02.
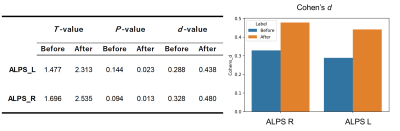
ComBat harmonization removed the scanner difference from multiscanner data and reduced the ALPS-index distribution in AD and CN. Consequently, the group difference after harmonization was approximately 1.5 times the original value, and the P-value also decreased, indicating that the statistical power (i.e., 1- ) improved. Thus, ComBat increased the statistical power of the multisite ALPS-index between CN and AD up to around two-fold.