3243
ComBat scanner harmonization for fixel-based analysis1Florey Institute of Neuroscience and Mental Health, Melbourne, Australia, 2Florey Department of Neuroscience and Mental Health, University of Melbourne, Melbourne, Australia, 3Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 4Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 5Department of Neurology, Austin Health, Melbourne, Australia, 6Auckland University of Technology, Auckland, New Zealand
Synopsis
Keywords: Data Analysis, Diffusion/other diffusion imaging techniques, Data harmonization
Diffusion MRI data are known to be sensitive to scanner and site effects, for which harmonization methods have been developed. However, harmonization methods have not yet been applied to many advanced diffusion imaging metrics. In this work, we apply the ComBat harmonization approach to fixel-based analysis. ComBat successfully minimizes scanner-related differences in fibre density and cross-section, and performs similarly to the inclusion of scanner as a nuisance regressor during whole-brain fixel-based analysis. Importantly, ComBat can now readily be used within the fixel-based framework, which will enable large multi-centre studies to implement this approach in the future.Introduction
Diffusion-weighted imaging (DWI) data are known to be sensitive to scanner and site effects, which can be a major limitation for multi-site or longitudinal studies1. Various data harmonization techniques have been developed to address this issue2,3, which can successfully minimize scanner- or site-related effects on DWI-based metrics. However, many of these harmonization methods have been only implemented on diffusion tensor imaging (DTI) metrics, and have not yet been implemented on other advanced DWI-based measures.Fixel-based analysis (FBA) is becoming a popular approach for DWI analysis, as it can provide fibre-specific estimates of white matter abnormality4. In this study, we implement the ComBat data harmonization approach2,5, a popular batch harmonization approach for imaging data, on fixel-level data (specific fibre populations within a voxel).
Methods
The MRI data included in this study was retrospective DWI data collected at our site on either a 3T Siemens Trio with a 12-channel head coil or 3T Siemens Skyra with a 20-channel head coil, using protocols that were designed to be equivalent. DWI data were acquired on the Trio with the following parameters: 60 axial slices, TR/TE = 8300/110 ms, 2.5 mm isotropic voxels, 60 diffusion-weighted images (b=3000 s/mm2) and 8 b=0 images. DWI data were acquired on the Skyra with the following parameters: 60 axial slices, TR/TE = 8400/110 ms, 2.5 mm isotropic voxels, 64 diffusion-weighted images (b=3000 s/mm2) and 8 b=0 images. A reverse phase-encoded b=0 image was acquired to correct for B0-field inhomogeneities.Participants included in this study were 90 neurologically healthy adults (mean age = 36 ± 13) scanned on either the Trio (n=23) or Skyra (n=67). We also included 21 patients with focal epilepsy due to focal cortical dysplasia (FCD; mean age = 33 ± 12) scanned either on the Trio (n=4) or Skyra (n=17), in whom whole-brain fixel-based findings have previously been examined6.
DWI data were preprocessed using MRtrix37. FODs were computed using single-shell 3-tissue constrained spherical convolution8, and spatial correspondence was achieved by creating a population template image from 40 randomly selected control individuals. Measures of fibre density and cross-section (FDC) were obtained at each white matter fixel and smoothed by fixel-based connectivity. We adapted the ComBat framework for the fixel image format to perform harmonization of fixel-based measures.
The following analyses were performed to test the ComBat approach:
- Whole-brain FBA9,10 comparing FDC measure in healthy control participants scanned on the Trio versus healthy control participants scanned on the Skyra, before and after ComBat
- Whole-brain FBA comparing FDC measure in focal epilepsy patients compared to healthy control participants, either: (i) with no adjustment for scanner; (ii) including scanner as a nuisance regressor; or (iii) adjusting FDC measures for scanner using ComBat
Results
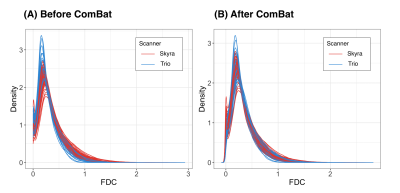
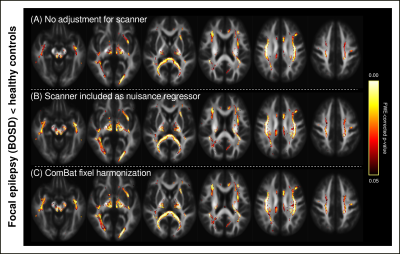
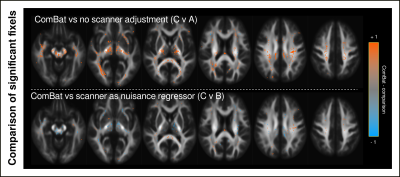
Figure 1 shows density plots comparing the fibre density and cross-section (FDC) measure for healthy control participants scanned on the Trio and Skyra, before and after ComBat. Whole-brain FBA revealed significant differences in FDC between control participants scanned on the Trio compared to those scanned on the Skyra, which were no longer present after ComBat harmonization (Figure 2).Figure 3 shows proof-of-principle results of whole-brain FBA comparing focal epilepsy patients to control participants, either (A) without adjustment for scanner differences, (B) including scanner as a nuisance regressor, or (C) following ComBat fixel harmonization. Although all three approaches exhibited regionally similar differences between the two groups, the ComBat approach recovered significant effects in many fixels that were not observed without adjustment for scanner, while significant fixels were similar in ComBat compared to the inclusion of scanner as a nuisance regressor (Figure 4).
A command has been created to perform ComBat on fixel data within the MRtrix3 framework called ‘fixelcombat’.
Discussion
In this work, we implement the ComBat data harmonization approach for fixel-based measures. We show that ComBat removes scanner-related differences in a fixel-based measure (fibre density and cross-section) within a healthy control cohort. We also show in a disease-related comparison, the ComBat approach recovers significant effects that are otherwise not observed without accounting for scanner differences. Of note, the ComBat approach performs similarly to the currently feasible alternative for fixel-based analysis, which is the inclusion of scanner as a nuisance regressor.Although the ComBat approach has previously been shown to be relatively robust to small sample sizes as low as 10-20 subjects per scanner2, this fixel-based harmonization approach should be validated using a larger cohort of epilepsy patients. Future work would also benefit from extending analyses to other neurological disorders. We also note that the analysis in this work is limited to two scanners from the same vendor with very similar diffusion acquisitions. Future work could explore the performance of ComBat alongside other diffusion harmonization approaches on data from diverse sources and acquisitions.
Importantly, ComBat can now readily be used within the FBA framework, which opens the possibility to implement this approach for wider use in multi-scanner and multi-site studies. This will potentially enable the fixel-based approach to be expanded from single-site studies with small sample sizes, to large multicentre studies in future.
Acknowledgements
MP is supported by an Emerging Grant Fellowship from the Health Research Council, New Zealand. RS is supported by fellowship funding from the National Imaging Facility (NIF), an Australian Government National Collaborative Research Infrastructure Strategy (NCRIS) capability. JDT's contribution was supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.References
1. Pinto, M. S. et al. Harmonization of Brain Diffusion MRI: Concepts and Methods. Front Neurosci 14, 396 (2020).
2. Fortin, J.-P. et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage 161, 149–170 (2017).
3. Mirzaalian, H. et al. Multi-site harmonization of diffusion MRI data in a registration framework. Brain Imaging Behav 12, 284–295 (2018).
4. Raffelt, D. A. et al. Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage 144, 58–73 (2017).
5. Fortin, J. P. et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage 167, 104 (2018).
6.
Mito, R., Vaughan, D. N., Semmelroch, M.,
Connelly, A. & Jackson, G. D. Bilateral Structural Network Abnormalities in
Epilepsy Associated With Bottom-of-Sulcus Dysplasia. Neurology 98,
e152–e163 (2022).
7. Tournier, J.-D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 551739 (2019) doi:10.1101/551739.
8. Dhollander, T. & Connelly, A. A novel iterative approach to reap the benefits of multi-tissue CSD from just single-shell (+ b= 0) diffusion MRI data. 24th International Society of Magnetic Resonance in Medicine 24, 3010 (2016).
9. Raffelt, D. A. et al. Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage 117, 40–55 (2015).
10. Smith, R. E. et al. Intrinsic non-stationarity correction for Fixel-Based Analysis. in Organisation for Human Brain Mapping (OHBM) M789 (2019).
Figures