3241
A Comprehensive Quality Assessment on Fast MRI Acquisition with united Compressed Sensing Techniques
Zhuoyang Gu1, Lianghu Guo1, Qing Yang1, Xinyi Cai1, Tianli Tao1, Sifan He1, Qian Wang1, He Qiang2, Dinggang Shen1, and Han Zhang1
1School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 2United Imaging Healthcare Co., Ltd., Shanghai, China
1School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 2United Imaging Healthcare Co., Ltd., Shanghai, China
Synopsis
Keywords: Parallel Imaging, Data Acquisition, Quality Assessment
MRI acquisition from certain populations is difficult. For instance, infant MRI without sedation during wakefulness has a high failure rate due to head motion. Thus, a shorter scan time is required. Increasing studies focused on fast MRI acquisition but few studies examined their qualities. We argue that only calculating common image quality metrics (e.g., SNR) may not meet the satisfaction of brain MRI analysis with fast acquisition techniques. A whole-process quality assessment method is thus proposed comparing different fast acquisition techniques with practical guidelines. In future, comprehensive quality assessment on fast MRI acquisitions can become a study routine.Introduction
MRI is a non-invasive and radiation-free diagnostic technology that plays a critical role in basic and clinical neuroscience research. However, collecting high-quality brain MRI data from certain populations (e.g., infants) is difficult, as they tend to move and cannot follow instructions to keep still for long scan time.1 Usually, MRI data with head motion artifacts has to be obsoleted, leading to significant data losses.Fast MRI acquisition techniques such as parallel imaging, compressive sensing (CS), and Half-Fourier (HF) can significantly shorten scan time,2 thus reducing motion artifacts and improving success rate and image quality. uCS (united compressed sensing) is a novel technique that combines parallel imaging, CS, and HF. Compared to conventional parallel imaging, which is often adopted in large-scale MRI database construction, uCS substantially shorten scan time without sacrificing image quality. Therefore, uCS has the potential to replace parallel imaging, overcoming the drawback of parallel imaging (i.e., prolonged scan time) in building high-resolution MRI databases. The performance of uCS needs further evaluation. In imaging quality assessment (QA), researchers often compare metrics such as contrast-to-noise ratio (CNR), signal-to-noise ratio (SNR), peak SNR (PSNR), structure similarity (SSIM), and sharpness.3 These metrics are often compared across different rates of acceleration to determine the optimal one.
However, we found that focusing on the aforementioned metrics derived from raw images may lead to misleading QA conclusions. The major issue is that QA metrics are only calculated from raw data, ignoring the goodness of image analysis results such as cortical thickness, cortical area, etc. It is essential for brain imaging research that “whole processing”-based QA should be conducted, with systematic evaluation of QA metrics derived from all processing steps. This is even more essential for development or aging studies where the brain is characterized by rapid and dynamic changes.4
We document a whole-processing-based, result-oriented, comprehensive QA method including visual inspection, quality metrics comparison, and statistical analysis on surface-based results. It could become a standard in future multisite, large-scale fast MRI acquisitions for big data research.
Methods
Twenty healthy adults (11 females aged 19–22 years) were scanned using a research-dedicated, United Imaging 3.0T uMR890 scanner equipped with uCS techniques. T1w FSP (Fast Spoiled gradient echo sequence) and T2w MATRIX (Modulated flip Angle Technique in Refocused Imaging with eXtended echo train) images were scanned from each subject with three protocols with different acquisition time (Fig. 1): paralleled imaging with acceleration factor 2 (P2), CS with acceleration factor 3 (uCS3), and uCS3.32. Image QA metrics were calculated by MRIQC5 and compared using paired t-tests for three protocols respectively.After calculating and comparing all the raw-image-based QA metrics, brain cortical surfaces were reconstructed using recon-all in Freesurfer6 and voxel/region-wise (DK atlas) surface geometric features (thickness, area, volume, and curvature) were extracted. Voxel-wise features were compared across different accelerating techniques using paired t-tests with cluster correction (p<0.05 corrected). Region-wise features were compared using ANOVA with post-hoc analysis (p<0.05 FWE corrected).
Results and Discussion
Visual inspection shows no apparent differences among different fast MRI acquisition techniques for T1w and T2w (Fig. 2a). Comparisons on the raw-image-based QA metrics show that uCS generally performs better than P2 and that uCS3.32 performs equally or even better than uCS3. The results of T2w images are similar to T1w images (Fig. 2b).Voxel-wise comparisons on cortical thickness show no significant differences between two uCS scans and P2 except for a small portion of the orbitofrontal region (possibly due to gradient-echo sequence in T1w, which is sensitive to B0 field inhomogeneity); no difference was found between uCS3 and uCS3.32 (Fig. 2c). Region-wise differences were spotted at several brain regions (Fig. 2d) but the relative changes are small (maximum 3.50% comparing P2 with uCS3.32). Other cortical features show similar results or have even smaller differences comparing different techniques.
Collectively, we found that uCS is a reliable technique, generating similar image-based analysis results with significantly shortened scan time compared with P2. When considering both data quality and success rate, we recommend uCS3.32 (scan time 44.6% less than P2). For studies concerning subtle changes in orbital frontal regions (i.e., studies on olfactory cortex), uCS could suffer a little bit more signal losses than P2 in this brain area. In this case, the result regarding this area should be interpreted with caution. To solve the signal loss issue, we further propose a deep learning-based image generation model, using T2w image generating less distorted T1w image. Initial results show satisfactory signal recovery ability (Fig. 3). Nevertheless, uCS introduces many benefits as demonstrated by our whole-process-based QA.
Conclusion
We provide a whole-process-based QA method to evaluate fast MRI acquisition technologies. We found comparable results can be obtained with uCS compared to P2. The conventional QA metrics are suggested not adequate to evaluate data quality with fast MRI acquisition techniques. Our proposed QA procedure on fast MRI acquisition techniques has been used in an ongoing infant development project for 0-6 years old infants.Acknowledgements
This work is partially supported by the National Key Technology R&D Program (Nos. 2022ZD0209000, 2021ZD0200516), Shanghai Pilot Program for Basic Research - Chinese Academy of Science, Shanghai Branch (No. JCYJ-SHFY-2022-014), Open Research Fund Program of National Innovation Center for Advanced Medical Devices (No. NMED2021ZD-01-001), Shenzhen Science and Technology Program (No. KCXFZ20211020163408012), Shanghai Pujiang Program (No. 21PJ1421400), and the National Key Technology R&D Program (Nos. 2022ZD0209000, 2021ZD0200516)References
1 Howell, B. R. et al. The UNC/UMN Baby Connectome Project (BCP): An overview of the study design and protocol development. NeuroImage 185, 891-905 (2019).2 Xiang, L. et al. Deep-learning-based multi-modal fusion for fast MR reconstruction. IEEE Transactions on Biomedical Engineering 66, 2105-2114 (2018).
3 Bazin, P.-L. et al. Sharpness in motion corrected quantitative imaging at 7T. NeuroImage 222, 117227 (2020).
4 Zhang, H., Shen, D. & Lin, W. Resting-state functional MRI studies on infant brains: a decade of gap-filling efforts. Neuroimage 185, 664-684 (2019).
5 Esteban, O. et al. MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites. PloS one 12, e0184661 (2017).
6 Fischl, B. FreeSurfer. Neuroimage 62, 774-781 (2012).
Figures
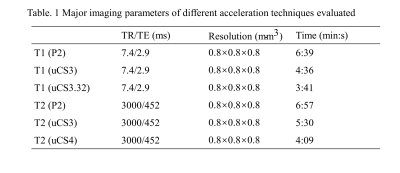
Fig. 1 Major imaging parameters of different acceleration techniques evaluated
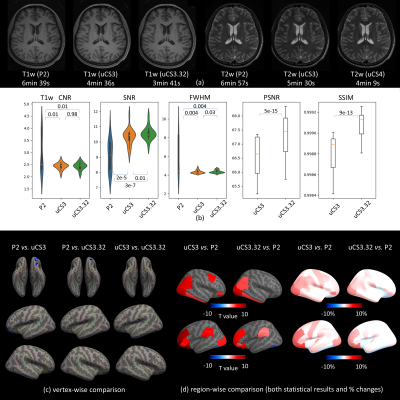
Fig. 2 Whole-process-based QC on fast MRI acquisition technologies. (a) T1w and T2w images from 3 protocols respectively. (b) Comparisons on various imaging quality metrics among 3 protocols with p values. (c) Vertex-wise surface measurement comparison (cortical thickness). (d) Paired t-test results and percentage of changes in region-wise surface measurement comparison between uCS and P2.
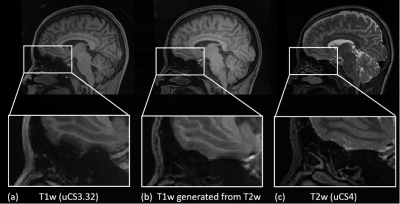
Fig. 3 T1w (uCS3.32), generated T1w, and T2w (uCS4) images.
DOI: https://doi.org/10.58530/2023/3241