3223
Magnetic Resonance Spectroscopic Correlates of Survival in IDH-wt, TERTp-mut Gliomas
Banu Sacli-Bilmez1, Ayça Erşen Danyeli2,3, Cengiz Yakicier4, M.Necmettin Pamir3,5, Koray Özduman3,5, Alp Dinçer3,6, and Esin Ozturk-Isik1,3
1Institute of Biomedical Engineering, Boğaziçi University, İstanbul, Turkey, 2Department of Medical Pathology, Acıbadem University, İstanbul, Turkey, 3Brain Tumor Research Group, Acıbadem University, İstanbul, Turkey, 4Department of Molecular Biology and Genetics, Acıbadem University, İstanbul, Turkey, 5Department of Neurosurgery, Acıbadem University, İstanbul, Turkey, 6Department of Radiology, Acıbadem University, İstanbul, Turkey
1Institute of Biomedical Engineering, Boğaziçi University, İstanbul, Turkey, 2Department of Medical Pathology, Acıbadem University, İstanbul, Turkey, 3Brain Tumor Research Group, Acıbadem University, İstanbul, Turkey, 4Department of Molecular Biology and Genetics, Acıbadem University, İstanbul, Turkey, 5Department of Neurosurgery, Acıbadem University, İstanbul, Turkey, 6Department of Radiology, Acıbadem University, İstanbul, Turkey
Synopsis
Keywords: Tumors, Brain
The 2021 central nervous system tumor classification has suggested that isocitrate dehydrogenase wildtype (IDH-wt) WHO grade-2/3 astrocytomas with molecular features of glioblastoma should be designated as “Glioblastoma, IDH-wildtype, WHO grade-4”. In this study, we explored metabolic correlates of patient survival in tumors fulfilling the criteria for “Glioblastoma, IDH-wildtype, WHO grade-4” using short echo time (TE) single voxel 1H-MRS. The results of this study suggest that higher ratios of glutamine and glutamate complex (Glx) to total creatine (tCr) and glutathione (GSH) to tCr were associated with a shorter survival.Introduction
A fraction of lower-grade (World Health Organization [WHO] grade 2 and 3) adult diffuse gliomas have bad prognosis [1, 2]. Recent molecular studies have shown that most of these cases are isocitrate dehydrogenase wildtype (IDH-wt) while having molecular features of glioblastoma, which are defined as the presence of the TERT-promoter (TERTp) mutation, EGFR gene amplification, and/or the combination of chromosome-7 gain and chromosome-10 loss (7+/10- pattern) [2, 3]. Consequently, the WHO 2021 central nervous system (CNS) tumor classification has suggested that IDH-wt WHO grade-2/3 astrocytomas with molecular features of glioblastoma should be designated as “Glioblastoma, IDH-wildtype, WHO grade-4” [4]. In this study, we explored metabolic correlates of patient survival in tumors fulfilling WHO 2021 CNS tumor classification criteria for “Glioblastoma, IDH-wildtype, WHO grade-4” using short echo time (TE) single voxel 1H-MRS.Methods
Fifty-seven IDH-wt, TERTp-mut adult diffuse glioma patients (35 men/22 women, mean age: 58 ± 10.47 years, median: 59 years, range: 39–75 years, 45 grade 4, eight grade 3, and four grade 2) were included in this study. The patients were scanned before surgery at a 3T Siemens scanner (Erlangen, Germany) using a 32-channel head coil. MRS data were acquired from the solid tumor region excluding necrosis, edema, and hemorrhage using a Point Resolved Spectroscopy (PRESS) sequence (TR/TE=2000/30 ms, voxel size=1-8 cm3). The patients were followed up for up to 65 months, and their overall (OS) and progression-free (PFS) survival times were recorded. LCModel spectral fitting program [5] was used for the quantification of MR spectroscopic peak concentrations of 19 metabolites including glycerophosphocholine (GPC), glutamate (Glu), glutamine (Gln), and glutathione (GSH), and five composite peak concentrations [total choline (tCho=GPC+PCh), total creatine (tCr=Cr+PCr), total N-acetyl aspartate (tNAA=NAA+NAAG), glutamine and glutamate complex (Glx), and myoinositol and glycine (mIns+Glyc)]. The metabolites, which have a Cramer-Rao lower bound (CRLB) of more than %30, were excluded from the study. The effects of metabolite concentrations on OS and PFS of IDH-wt, TERTp-mut glioma patients were evaluated using Kaplan–Meier survival analysis, followed by a log-rank test. Then, the predictive power of metabolic parameters, as well as age, sex, histological grade, contrast enhancement, and radiological necrosis, on OS and PFS were analyzed using classification and regression tree (CART) analysis. Kaplan-Meier analysis was performed using MATLAB 2021a (MathWorks, Natick, MA) while CART analysis was performed using SPSS statistics 25 (IBM Corp., Armonk, NY).Results
Kaplan–Meier analysis indicated that a higher Glx/tCr was associated with a significantly shorter PFS (p = 0.004) and a significantly shorter OS (p = 0.009; Figure 1A, 1B). A higher GSH/tCr ratio was also associated with a significantly shorter PFS (p = 0.005; Figure 1C). There was no statistically significant relationship between GSH/tCr ratio and OS (p = 0.356; Figure 1D). The results of the CART analysis of “IDH-wt, TERTp-mut diffuse gliomas” suggested that the patients with lower GSH/tCr (≤0.449) and lower Glx/tCr (≤1.474) had a better PFS while patients with higher GSH/tCr (>0.449) had the worst PFS (Figure 2A). High Glx/tCr (>2.966) was associated with a significantly shorter OS (Figure 2B).Discusion and Conclusion
The results of this study indicated that Glx/tCr and GSH/tCr were associated with shorter survival. Glu and Gln concentrations were reported to be correlated with tumor grade in diffuse gliomas [6]. Elevated GSH level has recently been indicated as an important biomarker of TERTp mutation in gliomas [7]. In conclusion, higher Glx/tCr and GSH/Cr ratios might be indicative of a more aggressive onco-metabolic profile and shorter survival in IDH-wt, TERTp-mut gliomas. These results will be validated on a larger patient cohort.Acknowledgements
This study has been supported by TUBITAK 1003 grant 216S432.References
- Eckel-Passow, J.E., et al., Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. The New England journal of medicine, 2015. 372(26): p. 2499-2508.
- Brat, D.J., et al., Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med, 2015. 372(26): p. 2481-98.
- Reuss, D.E., et al., Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol, 2015. 130(3): p. 407-17.
- Louis, D.N., et al., The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol, 2021. 23(8): p. 1231-1251.
- Provencher, S.W., Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed, 2001. 14(4): p. 260-4.
- Nagashima, H., et al., Myo-inositol concentration in MR spectroscopy for differentiating high grade glioma from primary central nervous system lymphoma. J Neurooncol, 2018. 136(2): p. 317-326.
- Viswanath, P., et al., Non-invasive assessment of telomere maintenance mechanisms in brain tumors. Nature Communications, 2021. 12(1): p. 92.
Figures
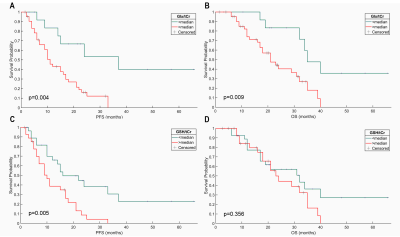
Kaplan–Meier curves of the effect of Glx/tCr (A-B) and GSH/tCr (C-D) on
the OS and PFS of IDH-wt, TERTp-mut glioma patients. The p values were obtained
from the log-rank test.
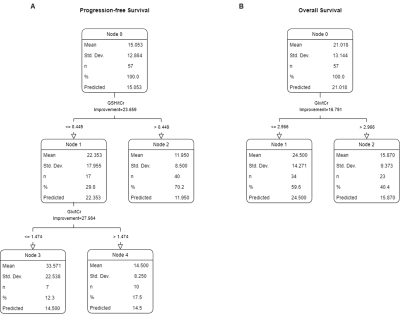
CART analysis for progression-free survival (A)
and overall survival (B) performed with clinical, anatomical, and metabolic
features of IDH-wt, TERTp-mut gliomas.
DOI: https://doi.org/10.58530/2023/3223