3221
Non-invasive Detection of IDH-mutant Gliomas using Single and Multi-voxel Point-resolved Spectroscopy1Radiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States, 2Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Neurosurgery, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States, 4Clinical Pathology and Laboratory Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States, 5Medicine, University of pennsylvania, Philadelphia, PA, United States
Synopsis
Keywords: Tumors, Spectroscopy, IDH mutant; gliomas; 2-hydroxyglutarate; SVS; 1H-MRSI
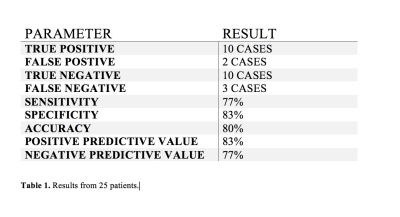
IDH mutation has become one of the most important prognostic biomarkers in glioma management, regardless of histopathological features. The oncometabolite 2HG has been proposed as a biomarker for IDH-specific genetic profiles for gliomas. We report clinical utility of SVS and 1H-MRSI using a long TE (97 ms) in assessing IDH-mutant gliomas by detecting the characteristic resonances of 2HG. Our results from 25 patients showed sensitivity and specificity of 77% and 83%, respectively. In conclusion, 1H-MRS with optimized TE can accurately detect 2HG levels, which has significant clinical implications for determining prognosis and evaluating therapeutic efficacy for targeted and/or alternative therapies.Introduction
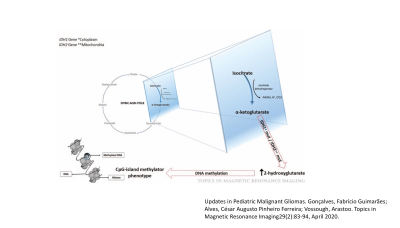
In the current WHO classification system, isocitrate dehydrogenase (IDH) mutation has become one of the most important prognostic biomarkers in glioma management, regardless of histopathology.1,2 Patients with glioma harboring IDH mutations demonstrate a better response to chemoradiation therapy and superior survival than those with IDH wild-type.3,4 While immunohistochemical analyses and exomic sequencing are considered as gold standards for determining IDH mutational status in gliomas,5,6 limited tissue availability and low tumor burden in tissue samples constraint the utility of these methods in reliable detection of IDH mutation status.7 Moreover, it may not always be possible to perform neurosurgical interventions because of the eloquent locations of these neoplasms. Therefore, non-invasive identification of IDH-mutant gliomas is important for making informed decisions on therapeutic intervention and for prognostication of these patients. Noteworthy, IDH mutations confer neomorphic activity of an enzyme leading to the conversion of alpha-ketoglutarate (α-KG) to 2-hydroxyglutarate (2HG) (Figure 1).8 The purpose of our study was to investigate the clinical utility of proton MR spectroscopy (1H-MRS) in identifying IDH-mutant gliomas by detecting characteristic resonances of 2HG.Methods
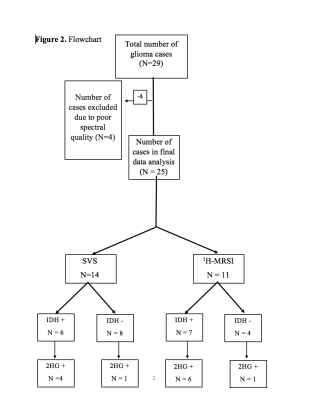
Twenty-nine patients with suspected intracranial neoplasms were recruited (Figure 2). These patients underwent anatomical imaging, single voxel (SVS, n=16) and /or single slice multivoxel magnetic resonance spectroscopic imaging (1H-MRSI, n=13) sequences on a 3T magnet. Both SVS (TR/TE/NEX=2000/97ms/128) and 1H-MRSI (TR/TE/NEX=2000/97ms/3) were acquired using standard spin-echo point-resolved spectroscopy (PRESS) sequence. For 1H-MRSI, the volume of interest (VOI) was selected to include neoplasm visible as hyperintense mass on T2-FLAIR images and contralateral normal parenchyma avoiding scalp, skull base, or sinuses. Eight outer volume saturation slabs were placed outside VOI to suppress lipid signals from scalp. The data set was acquired using elliptical-K-space sampling with weighted phase encoding. Manual shimming was performed to achieve an optimal fullwidth at half maximum (FWHM) value (<20 Hz) of water signal. Both water-suppressed and unsuppressed spectra were acquired, and the unsuppressed water signal was used for computing metabolite concentrations. Of these 29 patients, data were excluded from 4 patients due to poor-spectral quality. All spectroscopy data were analyzed using a user-independent spectral fit program [linear combination (LC) model].9 The region between 0.2 and 4.2 ppm of the spectrum was analyzed, and the following metabolites were evaluated: N-acetylaspartate (NAA), 2.02 ppm; creatine (Cr), 3.02ppm; choline (Cho), 3.22 ppm; glutamate+glutamine (Glx), 2.24 to 2.34 ppm; myo-inositol (mI), 3.56 ppm and 2HG, (2.25ppm). The quality of spectral fitting was evaluated by analyzing difference spectrum (fitted spectrum subtracted from original spectrum) and by the Cramer-Rao lower bound (CRLB) values, with less than 40% considered acceptable for detecting 2HG10 and less than 20% for all other metabolites.11,12 Histopathological and immunohistochemical analyses for glioma grade and IDH mutation status were subsequently performed from resected tumor specimens and the findings were compared with the results from spectral data. Absolute quantification of 2HG and metabolite ratio of 2HG/Cr were computed from true positive IDH-mutant gliomas.Results
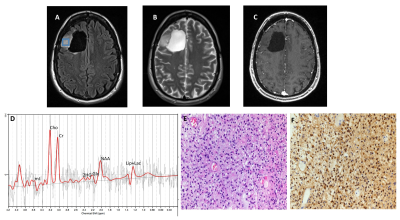
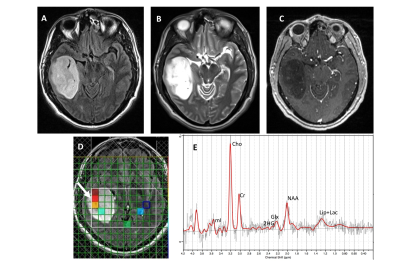
A total of 25 patients were included (mean age = 47.6 ± 15 years, 10 males and 15 females) in the final data analysis. All tumors were histopathologically confirmed for gliomas. 11/14 (79%) cases were correctly identified as IDH-mutant or IDH-wildtype gliomas by SVS and 9/11 (82%) by 1H-MRSI (Figures 3 and 4), with an overall concordance rate of 80% (20/25). Three patients were scanned using both techniques and were correctly identified, being two of them IDH-mutant. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) in identifying IDH-mutant and IDH-wildtype gliomas were 77%, 83%, 83% and 77%, respectively (Table 1). The mean concentration of 2HG and metabolite ratio of 2HG/Cr in IDH-mutant cases were 3.95 ±3.29 mM and 0.64±0.33, respectively.Discussion
We report the clinical utility of SVS and 1H-MRSI using an optimized TE (97ms) in assessing IDH-mutant gliomas by detecting the characteristic resonances of 2HG. Mechanistically, wildtype IDH normally catalyzes the reversible NADP+ dependent oxidative decarboxylation of isocitrate to α-KG in the TCA cycle. Nevertheless, IDH mutations confer a neomorphic enzyme activity converting α-KG to 2HG. The oncometabolite 2HG has been proposed as a putative biomarker for IDH-mutation-specific genetic profiles for gliomas. Some previous studies have employed sophisticated spectroscopic sequences and post-processing tools for detecting spectral resonances of 2HG from IDH-mutant gliomas.13–17 Earlier, we have shown the potential of using two-dimensional localized correlation spectroscopy (2D L-COSY) at 7 Tesla to detect 2-HG in IDH-mutant gliomas.13 However, sophisticated spectroscopic sequences, such as 2D L-COSY, are not readily available in routine clinical settings. In this study, we showed that SVS and 1H-MRSI sequences with an optimized TE of 97ms on a 3T scanner were able to resolve complex resonances of 2HG from Glx and GABA and detect 2HG in IDH-mutant gliomas with high accuracy.Conclusion
We have demonstrated that 1H-MRS with optimized echo-time (97ms) may be useful to detect 2HG in -mutant glioma patients, which has significant clinical implications for determining prognosis and evaluating therapeutic efficacies of targeted and/or alternative therapies.Acknowledgements
NoneReferences
1. Louis, D. N. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 131, 803–820 (2016).
2. Louis, D. N. et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-Oncology vol. 23 1231–1251 (2021).
3. Yan, W. et al. Correlation of IDH1 Mutation with Clinicopathologic Factors and Prognosis in Primary Glioblastoma: A Report of 118 Patients from China. PLoS One 7, e30339 (2012).
4. Zhang, C.-B. et al. Correlation of IDH1/2 Mutation with Clinicopathologic Factors and Prognosis in Anaplastic Gliomas: A Report of 203 Patients from China. Journal of Cancer Research and Clinical Oncology vol. 140 45–51 (2014).
5. Parsons, D. W. et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science vol. 321 1807–1812 (2008).
6. Yan, H. et al. IDH1 and IDH2 Mutations in Gliomas. New England Journal of Medicine vol. 360 765–773 (2009).
7. Preusser, M. et al. Value and Limitations of Immunohistochemistry and Gene Sequencing for Detection of the IDH1-R132H Mutation in Diffuse Glioma Biopsy Specimens. Journal of Neuropathology & Experimental Neurology vol. 70 715–723 (2011).
8. Dang, L. et al. Cancer-associated IDH1 Mutations Produce 2-hydroxyglutarate. Nature vol. 462 739–744 (2009).
9. Provencher, S. W. Estimation of Metabolite Concentrations from Localized in vivo Proton NMR Spectra. Magnetic Resonance in Medicine vol. 30 672–679 (1993).
10. Batsios, G. et al. PI3K/mTOR Inhibition of IDH1 Mutant Glioma Leads to Reduced 2HG Production that is Associated with Increased Survival. Sci. Rep.9, 10521 (2019).
11. Kim, H., Kim, S., Lee, H. H. & Heo, H. In-Vivo Proton Magnetic Resonance Spectroscopy of 2-Hydroxyglutarate in Isocitrate Dehydrogenase-Mutated Gliomas: A Technical Review for Neuroradiologists. Korean Journal of Radiology vol. 17 620 (2016).
12. Tietze, A. et al. Noninvasive Assessment of Isocitrate Dehydrogenase Mutation Status in Cerebral Gliomas by Magnetic Resonance Spectroscopy in a Clinical Setting. Journal of Neurosurgery vol. 128 391–398 (2018).
13. Verma, G. et al. Non-invasive Detection of 2-Hydroxyglutarate in IDH-mutated Gliomas Using Two-Dimensional Localized Correlation Spectroscopy (2D L-COSY) at 7 Tesla. Journal of Translational Medicine vol. 14 (2016).
14. Askari, P. et al. Spectral Fitting Strategy to Overcome the Overlap Between 2‐Hydroxyglutarate and Lipid Resonances at 2.25 ppm. Magnetic Resonance in Medicine vol. 86 1818–1828 (2021).
15. Choi, C. et al. Prospective Longitudinal Analysis of 2-Hydroxyglutarate Magnetic Resonance Spectroscopy Identifies Broad Clinical Utility for the Management of Patients With IDH-Mutant Glioma. Journal of Clinical Oncology vol. 34 4030–4039 (2016).
16. Gutman, D. A. et al. Somatic mutations associated with MRI-derived volumetric features in glioblastoma. Neuroradiology vol. 57 1227–1237 (2015).
17. An, Z. et al. Detection of 2-Hydroxyglutarate in Brain Tumors by Triple-refocusing MR Spectroscopy at 3T in vivo. Magnetic Resonance in Medicine vol. 78 40–48 (2017).
Figures