3217
Impact of gadopiclenol on decision making in patients with brain metastases
Marco Essig1, Frank A Giordano2, and Gustavo Sarria3
1Radiology, University of Manitoba, Winnipeg, MB, Canada, 2Radiation Oncology, University of Mannheim, Mannheim, Germany, 3Radiation Oncology, University of Bonn, Bonn, Germany
1Radiology, University of Manitoba, Winnipeg, MB, Canada, 2Radiation Oncology, University of Mannheim, Mannheim, Germany, 3Radiation Oncology, University of Bonn, Bonn, Germany
Synopsis
Keywords: Tumors, Contrast Agent, Brain Tumor, Relativity
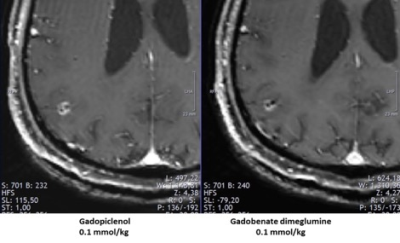
New gadolinium-based contrast agents (GBCAs) with high relaxivity could have an impact on decision making and treatment planning of brain metastases (BM). In this post-hoc analysis , patients with BM who underwent two separate magnetic resonance imaging (MRI) examinations, one with gadopiclenol and one with gadobenate dimeglumine, both at 0.1 mmol/kg, were included. This study showed that gadopiclenol at the dose of 0.1 mmol/kg improved detection and characterization of BM, and led to a change in treatment decisions in 2 of 13 patients.Introduction
Gadopiclenol 1 (Elucirem™, Guerbet, France) is a high relaxivity macrocyclic GBCA registered in US and currently under review by EU regulatory authorities. This study aimed to evaluate the impact of contrast-enhanced MRI with gadopiclenol on decision making and radiotherapy (RT) treatment planning of BM.
Methods
This is a post-hoc analysis of data from a phase IIb study 2 where patients underwent two separate MRI examinations, one with gadopiclenol at different doses (0.025, 0.05, 0.1, 0.2 mmol/kg) and one with gadobenate dimeglumine at 0.1 mmol/kg. Patients who received gadopiclenol and gadobenate dimeglumine, both at 0.1 mmol/kg, with ≥1 BM detected in any of both scans, were subjected to a blinded reader analysis and contouring. For each patient, treatment plans (stereotactic radiosurgery [SRS] or whole-brain radiotherapy [WBRT]) were determined for both MRIs, with the gross target volume (GTV) indicating the contrast-enhancing aspects of the tumor. Mean GTVs and normal tissue volumes receiving 12 Gy (V12), as well as the Dice similarity coefficient (DSC) were obtained for the paired contours. The Spearman´s rank (ρ) correlation was additionally calculated. Furthermore, three experts blindly evaluated the contrast enhancement of each lesion for contouring purposes and subjectively qualified them as “better”, in detriment of the counterpart, or “equal”.
Results
A total of 13 adult patients (69% males) presenting with at least one BM were analyzed. Gadopiclenol depicted additional BM as compared with gadobenate dimeglumine in 7 patients (54%). Treatment indication was changed in 2 patients (15%), from no treatment to SRS and from SRS to WBRT. The mean GTVs and V12 were comparable between gadopiclenol and gadobenate dimeglumine (p=0.694, p=1.974). The mean DSC was 0.70 (± 0.14, ρ 0.82). From a total of 36 answers, an improvement in enhancement was qualified in 21 (58.3%) with gadopiclenol, 8 (22.2%) with gadobenate dimeglumine, while no difference was obtained in 7 (19.4%) evaluations.DiscussionThe improved lesion identification and characterization of BM with gadopiclenol at 0.1 mmol/kg, could be explained by its doubled relaxivity as compared to gadobenate dimeglumine, therefore providing better BM contrast enhancement.
Conclusion
Gadopiclenol at 0.1 mmol/kg improved BM detection and characterization with impact on RT treatment decisions. Further trials are warranted to determine the impact of this MRI contrast agent on a clinical setting.
Gadopiclenol 1 (Elucirem™, Guerbet, France) is a high relaxivity macrocyclic GBCA registered in US and currently under review by EU regulatory authorities. This study aimed to evaluate the impact of contrast-enhanced MRI with gadopiclenol on decision making and radiotherapy (RT) treatment planning of BM.
Methods
This is a post-hoc analysis of data from a phase IIb study 2 where patients underwent two separate MRI examinations, one with gadopiclenol at different doses (0.025, 0.05, 0.1, 0.2 mmol/kg) and one with gadobenate dimeglumine at 0.1 mmol/kg. Patients who received gadopiclenol and gadobenate dimeglumine, both at 0.1 mmol/kg, with ≥1 BM detected in any of both scans, were subjected to a blinded reader analysis and contouring. For each patient, treatment plans (stereotactic radiosurgery [SRS] or whole-brain radiotherapy [WBRT]) were determined for both MRIs, with the gross target volume (GTV) indicating the contrast-enhancing aspects of the tumor. Mean GTVs and normal tissue volumes receiving 12 Gy (V12), as well as the Dice similarity coefficient (DSC) were obtained for the paired contours. The Spearman´s rank (ρ) correlation was additionally calculated. Furthermore, three experts blindly evaluated the contrast enhancement of each lesion for contouring purposes and subjectively qualified them as “better”, in detriment of the counterpart, or “equal”.
Results
A total of 13 adult patients (69% males) presenting with at least one BM were analyzed. Gadopiclenol depicted additional BM as compared with gadobenate dimeglumine in 7 patients (54%). Treatment indication was changed in 2 patients (15%), from no treatment to SRS and from SRS to WBRT. The mean GTVs and V12 were comparable between gadopiclenol and gadobenate dimeglumine (p=0.694, p=1.974). The mean DSC was 0.70 (± 0.14, ρ 0.82). From a total of 36 answers, an improvement in enhancement was qualified in 21 (58.3%) with gadopiclenol, 8 (22.2%) with gadobenate dimeglumine, while no difference was obtained in 7 (19.4%) evaluations.DiscussionThe improved lesion identification and characterization of BM with gadopiclenol at 0.1 mmol/kg, could be explained by its doubled relaxivity as compared to gadobenate dimeglumine, therefore providing better BM contrast enhancement.
Conclusion
Gadopiclenol at 0.1 mmol/kg improved BM detection and characterization with impact on RT treatment decisions. Further trials are warranted to determine the impact of this MRI contrast agent on a clinical setting.
Acknowledgements
No acknowledgement found.References
1- Robic C, Port M, Rousseaux O, et al. Physicochemical and Pharmacokinetic Profiles of Gadopiclenol: A New Macrocyclic Gadolinium Chelate With High T1 Relaxivity. Invest Radiol. 2019 Aug;54(8):475-484.2- Bendszus M, Roberts D, Kolumban B, et al. Dose Finding Study of Gadopiclenol, a New Macrocyclic Contrast Agent, in MRI of Central Nervous System. Invest Radiol. 2020 Mar;55(3):129-137.
DOI: https://doi.org/10.58530/2023/3217