3212
Neuromelanin hemispheric asymmetry in normal aging and early Parkinson’s disease with motor symptom laterality
Xueling Liu1, Yuxin Li1, Puyeh Wu2, Na Wang1, Fengtao Liu3, and Daoying Geng1
1Radiology Department of Huashan Hospital affiliated to Fudan University, Shanghai, China, 2GE healthcare, Beijing, China, 3Neurology Department of Huashan Hospital affiliated to Fudan University, Shanghai, China
1Radiology Department of Huashan Hospital affiliated to Fudan University, Shanghai, China, 2GE healthcare, Beijing, China, 3Neurology Department of Huashan Hospital affiliated to Fudan University, Shanghai, China
Synopsis
Keywords: Parkinson's Disease, Neurodegeneration, neuromelanin
The bilateral neuromelanin degeneration pattern in substantia nigra in normal aging and underlying mechanisms of asymmetric motor symptoms in early Parkinson’s disease (PD) are still unknown. Here we examined the neuromelanin hemispheric asymmetry in normal elderly people and early PD patients with motor symptoms laterality by short-echo-time magnitude images derived from QSM. We demonstrated hemispheric asymmetry of neuromelanin in normal aging, as well as a left-hemispheric preferential nigral dysfunction in early-stage PDR, but a different spatial neuromelanin loss pattern in PDL. This template-based neuromelanin measurement might help to detect aging-related change and deepen our understanding of PD with motor laterality.Introduction
Parkinson’s disease (PD) is a progressive neurodegeneration disorder, and its cardinal motor symptoms are bradykinesia, rigidity, resting tremor, and postural instability1, which often present unilaterally2-5. The asymmetric feature of motor symptoms implied the possibility of lateralized dysfunction of the nigrostriatal circuit.However, laterality of dopaminergic neurons in the substantia nigra (SN) has yet been fully investigated. Several MRI markers are able to detect underlying neuroanatomic, functional and pathophysiologic alterations in SN6. Neuromelanin (NM)-sensitive MRI7 could visualize the hyperintensity areas containing neuromelanin, a by-product of dopamine and noradrenaline metabolism. One study reported the concordance between motor asymmetry and NM changes of SNc was 61.36%4. Another study revealed lower nigral pigmentation in the clinically-defined most affected side, and relationships between nigral pigmentation and striatal dopamine transporter binding were only observed in the severity side8. Notably, PD with right-side dominance (PDR) was associated with a faster disease progression5 and more medication-related motor fluctuations9, while PDL was associated with longer disease duration9 and more impairment of non-motor symptoms10-12. It is necessary to explore similarities and differences of nigral dysfunction in PDR and PDL, as well as bilateral degeneration pattern in normal aging.
In this study, we aimed to investigate the neuromelanin hemispheric asymmetry in normal elderly people and early-PD patients with motor symptoms laterality by short-echo-time magnitude (setMag) images derived from QSM13. Primarily, we focused on both hemisphere effect and group effect, then compared the neuromelanin loss ratio between the most and the least affected side in PDR and PDL.
Methods
Ethical approval was obtained from our hospital (No. KY2018-226). Informed consent was obtained from each participant. 52 patients with PD and 34 healthy controls (HC) were recruited in this study. In PD group, H&Y scale14 was evaluated and only early-stage patients with HY stage 1 and 2 were included. The severity of motor dysfunction was assessed based on the MDS-UPDRS Part III15, and we calculated the sub-score of rigidity, bradykinesia and tremor16. Only patients with definite motor asymmetry (at least 2 points difference) were included.MR examinations were performed on a 3.0-T MR scanner (Signa HDxt; GE Healthcare, Milwaukee, WI). QSM images were acquired with a 3D multi-echo GRE sequence (TR/TE = 41.6/3.2:2.4:38.5 ms; flip angle = 12°; FOV = 256 × 256 mm; matrix = 256 × 256; thickness = 1 mm). NM-sensitive setMag images were reconstructed from QSM data following the procedure published previously13.
We firstly constructed a setMag template based on the symmetric group-wise normalization (SyGN) method using advanced normalization tools (ANTs)17,18. Bilateral hyperintensity of SNc areas were manually outlined on the setMag template using ITK-SNAP19, and then segmented into three ROIs: posterolateral sensorimotor, anteromedial associative, and posteromedial limbic regions16,20,21. Bilateral cerebral peduncle (CP) masks were also defined as a reference region. Subsequently, these masks were applied to each participant’s setMag image (Figure 1). The neuromelanin contrast ratio (CR) was calculated as follows7: $$CR = (SNCROI/CPROI)-1$$
where SNCROI and CPROI indicate signal intensity in SNc and CP, respectively. Neuromelanin loss ratio (LR) was calculated as follows: $$LR = (1-CRPD/CRHC)*100%$$
where CRPD and CRHC indicate contrast ratio in PD and the mean contrast ratio in HC on the bilateral territories of SNc, respectively.
Comparisons of demographic information and clinical characteristics were conducted using one-way ANOVA, independent t-test, and Chi-square test among groups. To assess hemispheric asymmetry differences of CR, a mixed ANOVA was conducted with group as between-subjects factors, hemisphere as within-subject factors, and age, gender as covariates. Paired-t test was conducted to compare LR differences between the most and least affected side.
Results
The clinical and demographic data are shown in Table 1. All groups were matched for age, sex and the clinical indexes.Mixed ANOVA showed a hemispheric asymmetry of CR in HC and PDR groups (Figure 2), while not in PDL group, where only a tendency of lower CR in the left sensorimotor territory was found (p = 0.055). Mixed ANOVA showed a significant group difference of CR in right and left hemispheres among three groups (Figure 3). In comparison to HCs, PDR patients showed significant reduced CR in the sensorimotor, associative and limbic subregions of SNc on both hemispheres. For PDL patients, CR were significantly reduced in the sensorimotor, associative and limbic subregions of right SNc, and only sensorimotor subregion of left SNc. For the comparison between PDR and PDL groups, CR was reduced in PDR patients in the left limbic subregion, while no differences were found in the right SNc.
In PDR patients, the LR were significantly higher in the most affected side compared to the least affected side in the sensorimotor and associative subregions of SNc, and had a lower tendency in the limbic subregion (Table 2). However, in PDL patients, the most affected side showed more NM loss in the associative and limbic subregions of SNc.
Conclusion
In conclusion, the current study demonstrates hemispheric asymmetry of neuromelanin in normal aging, as well as a left-hemispheric preferential nigral dysfunction in PDR, but a different spatial neuromelanin loss pattern in PDL. This template-based neuromelanin measurement might help to detect aging-related change and deepen our understanding of PD with motor laterality.Acknowledgements
Authors thank Yinan Sun for her support in beautify
the figures. Authors thank all patients and healthy controls in this study. All
authors declare no conflicts of interest.
References
1 Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society 30, 1591-1601, doi:10.1002/mds.26424 (2015).2 Toth, C., Rajput, M. & Rajput, A. H. Anomalies of asymmetry of clinical signs in parkinsonism. Movement disorders : official journal of the Movement Disorder Society 19, 151-157, doi:10.1002/mds.10685 (2004).3 Uitti, R. J., Baba, Y., Whaley, N. R., Wszolek, Z. K. & Putzke, J. D. Parkinson disease: handedness predicts asymmetry. Neurology 64, 1925-1930, doi:10.1212/01.Wnl.0000163993.82388.C8 (2005).4 Prasad, S., Saini, J., Yadav, R. & Pal, P. K. Motor asymmetry and neuromelanin imaging: Concordance in Parkinson's disease. Parkinsonism & related disorders 53, 28-32, doi:10.1016/j.parkreldis.2018.04.022 (2018).5 Baumann, C. R., Held, U., Valko, P. O., Wienecke, M. & Waldvogel, D. Body side and predominant motor features at the onset of Parkinson's disease are linked to motor and nonmotor progression. Movement disorders : official journal of the Movement Disorder Society 29, 207-213, doi:10.1002/mds.25650 (2014).6 Bae, Y. J. et al. Imaging the Substantia Nigra in Parkinson Disease and Other Parkinsonian Syndromes. Radiology 300, 260-278, doi:10.1148/radiol.2021203341 (2021).7 Sasaki, M. et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease. Neuroreport 17, 1215-1218, doi:10.1097/01.wnr.0000227984.84927.a7 (2006).8 Martin-Bastida, A. et al. Relationship between neuromelanin and dopamine terminals within the Parkinson's nigrostriatal system. Brain : a journal of neurology 142, 2023-2036, doi:10.1093/brain/awz120 (2019).9 Munhoz, R. P. et al. Long-duration Parkinson's disease: role of lateralization of motor features. Parkinsonism & related disorders 19, 77-80, doi:10.1016/j.parkreldis.2012.07.008 (2013).10 Huang, P. et al. Motor-symptom laterality affects acquisition in Parkinson's disease: A cognitive and functional magnetic resonance imaging study. 32, 1047-1055, doi:10.1002/mds.27000 (2017).11 Lee, E. Y. et al. Side of motor onset is associated with hemisphere-specific memory decline and lateralized gray matter loss in Parkinson's disease. Parkinsonism & related disorders 21, 465-470, doi:10.1016/j.parkreldis.2015.02.008 (2015).12 Zhu, S. et al. The Association Between Clinical Characteristics and Motor Symptom Laterality in Patients With Parkinson's Disease. Frontiers in neurology 12, 663232, doi:10.3389/fneur.2021.663232 (2021).13 Liu, X. et al. Short-echo-time magnitude image derived from quantitative susceptibility mapping could resemble neuromelanin-sensitive MRI image in substantia nigra. 20, 262, doi:10.1186/s12883-020-01828-8 (2020).14 Goetz, C. G. et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Movement disorders : official journal of the Movement Disorder Society 19, 1020-1028, doi:10.1002/mds.20213 (2004).15 Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders : official journal of the Movement Disorder Society 23, 2129-2170, doi:10.1002/mds.22340 (2008).16 Biondetti, E. et al. Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson's disease. Brain : a journal of neurology 143, 2757-2770, doi:10.1093/brain/awaa216 (2020).17 Avants, B. B. et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage 49, 2457-2466, doi:10.1016/j.neuroimage.2009.09.062 (2010).18 Yu, B. et al. HybraPD atlas: Towards precise subcortical nuclei segmentation using multimodality medical images in patients with Parkinson disease. Human brain mapping 42, 4399-4421, doi:10.1002/hbm.25556 (2021).19 Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116-1128, doi:10.1016/j.neuroimage.2006.01.015 (2006).20 Biondetti, E. et al. The spatiotemporal changes in dopamine, neuromelanin and iron characterizing Parkinson's disease. Brain : a journal of neurology 144, 3114-3125, doi:10.1093/brain/awab191 (2021). 21 Chougar, L. et al. Regional Selectivity of Neuromelanin Changes in the Substantia Nigra in Atypical Parkinsonism. Movement disorders : official journal of the Movement Disorder Society 37, 1245-1255, doi:10.1002/mds.28988 (2022).Figures
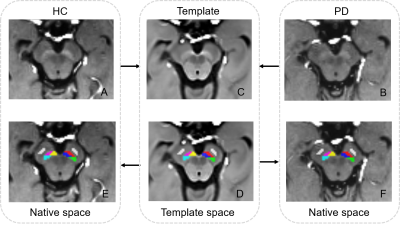
SetMag
images template and ROIs overlay.
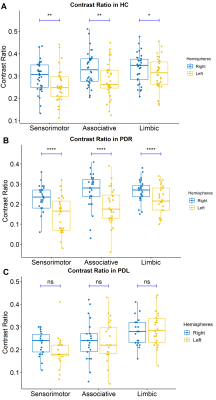
Hemispheric differences of neuromelanin contrast ratio
in the sensorimotor, associative and limbic subregions
of SNc in HC (A), PDR (B) and PDL (C) groups.
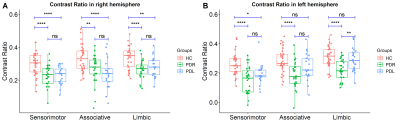
Group
differences of neuromelanin loss in HC, PDR and PDL groups in right (A) and
left (B) hemispheres in the sensorimotor, associative and limbic subregions of
SNc.

Hemispheric and group differences of neuromelanin contrast ratios of SNc in HC, PDR
and PDL groups
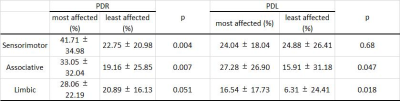
Comparison
of neuromelanin loss ratio
between the most and least affected side in PDR and PDL
DOI: https://doi.org/10.58530/2023/3212