3207
Differential Diagnosis Between Parkinson's Disease and Essential Tremor with Neuromelanin Imaging of the Substantia Nigra and Locus Coeruleus
Xinhui Wang1, Naying He1, Ewart Mark Haacke2, Yu Liu1, Peng Liu1, Youmin Zhang1, Zhijia Jin1, Yan Li1, Peng Wu3, and Fuhua Yan1
1Radiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 2Biomedical Engineering, Wayne State University, Detroit, MI, United States, 3Philips Healthcare, Shanghai, China
1Radiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 2Biomedical Engineering, Wayne State University, Detroit, MI, United States, 3Philips Healthcare, Shanghai, China
Synopsis
Keywords: Parkinson's Disease, Brain, essential tremor; neuromelanin MRI; locus coeruleus;substantia nigra
In this study, we used neuromelanin MRI to investigate locus coeruleus (LC) and substantia nigra changes between essential tremor (ET) and Parkinson’s disease (PD). We found the neuromelanin of LC was significantly lower in tremor-dominant PD patients than in ET patients or healthy controls, and the area under the curve of the model constructed by NM measures reached 0.92 in differentiating tremor-dominant PD from ET. These results provided new perspectives in the differential diagnosis of patients with tremor and in the investigation of the underlying pathophysiology.Introduction
Differential diagnosis of essential tremor (ET) and Parkinson’s disease (PD) (the two most common movement disorders with tremor) can still be a challenge in clinical practice. These two tremor disorders may have different pathogenesis related to the substantia nigra (SN) and locus coeruleus (LC). Characterizing neuromelanin (NM) in these structures may help improve the differential diagnosis. This study aimed to explore the combined value of NM measures in the LC and SN in the differentiation of tremor-dominant PD (PDTD) from ET patients as extracted from NM magnetic resonance imaging (NM-MRI) data.Methods
Forty-three patients with PDTD, 31 patients with ET, and 30 age- and sex-matched healthy controls were included. All subjects were scanned with a 3D multi-echo gradient recalled echo sequence with an activated magnetization transfer contrast pulse on a 3.0 T Philips Ingenia scanner. The imaging parameters were as follows: voxel size = 0.67×1×1.34 mm3 interpolated to 0.67×0.67×1.34 mm3, first echo time (TE) = 7.5ms, (with 4 more echoes each with a separation of 7.5ms), repeat time (TR) = 62ms, flip angle =30° and 12°, pixel bandwidth =174 Hz/pixel, slice thickness =2 mm, number of slices = 48, a sense factor of 2, and a total acquisition time of 9 minutes 20 seconds inclusive of both flip angles. The NM regions of interest (ROIs) associated with the SN were automatically drawn on the 12° flip angle original transverse images using SPIN software (SpinTech, Inc., Bingham Farms, Ml, USA). The NM ROIs for LC were manually segmented on each subject from the 30° MTC magnitude coronally reformatted images. The background ROIs for the SN and LC were set in nearby structures. The NM volume and contrast-to-noise ratio (CNR) of the SN, and NM area and CNR of LC derived from the NM-MRI data were evaluated (Figure 1). Logistic regression was used to calculate predicted probabilities by using a combination of SN and LC NM parameters. The discriminative power of the NM measures in detecting patients with PDTD from ET was assessed with a receiver operative characteristic curve.Results
The NM CNR of the LC, the NM volume, and the CNR of the SN on the right and left sides were significantly lower in PDTD patients than in ET patients or healthy controls (all P<0.05) (Figure2). Furthermore, when combining the best model constructed from the NM measures, the AUC reached 0.92 in differentiating PDTD from ET (Figure3).Discussion
The reduced NM parameters in PDTD compared to ET is similar to what has been found in a previous study1. In our analysis, the NM CNR calculation of SN focused on the central part of the SN, because it was difficult to find the lateral part of the SN in all slices, especially in PDTD patients. In addition, a previous study suggested that noradrenergic neurons in ET were not diminished in the LC when compared with healthy controls2. Our study is similar to the pathological study, we found no significant difference for LC NM CNR between healthy controls and patients with ET.Conclusion
The NM volume and contrast in both the LC and SN derived from NM-MRI could be a potential diagnostic biomarker to differentiate PDTD from ET.Acknowledgements
NoneReferences
1. Reimão S, Pita Lobo P, Neutel D, et al. Substantia Nigra Neuromelanin-MR Imaging Differentiates Essential Tremor From Parkinson’s Disease. Mov Disord 2015;30(7):953-959.
2. Shill HA, Adler CH, Beach TG, et al. Brain biochemistry in autopsied patients with essential tremor. Mov Disord 2012;27(1):113-117.
Figures
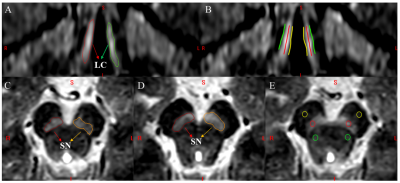
Figure 1. The locus coeruleus (LC) and substantia nigra (SN) as visualized in neuromelanin (NM) sensitive MRI. (A) The regions of interest (ROIs) of LC. (B) The reference lines for measuring the contrast of LC. (C, D) The ROIs for the NM in SN. (E) The contrast in SN. Green lines and yellow lines in B: Reference lines of the background of LC; Red lines in B: Bilateral LC; Yellow circles on E: Reference regions anterior to each SN ROI; Green circles on E: Reference regions posterior to each SN ROI but not on red nuclei; Red circles on E: Bilateral SN.
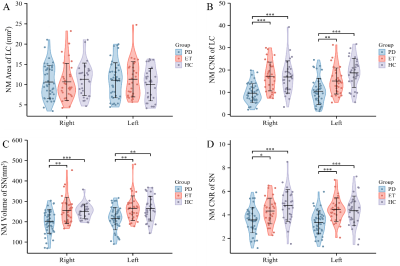
Figure 2. NM-MRI parameters of the LC and SN were assessed in patients with ET, PDTD and HCs. (A) The LC NM area for both right and left sides: LC area of PDTD (blue), ET (red), and HC (purple); (B) The LC NM CNR on both right and left sides of three groups; (C) SN NM volume on the right and left sides and (D) the SN NM CNR on both right and left sides (D). NM-MRI=Neuromelanin MRI; LC=locus coeruleus; SN = substantia nigra; PDTD = Tremor-dominant Parkinson’s disease; ET = Essential tremor; HC = Healthy controls; CNR = contrast-to-noise ratio. *P<0.05, **P <0.01, ***P < 0.001.
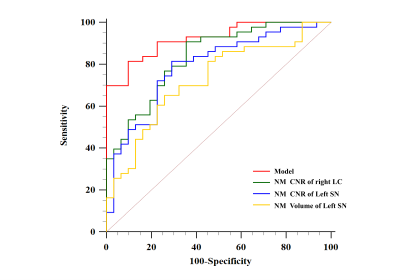
Figure 3. Receiver operative characteristic curve for neuromelanin (NM) contrast-to-noise ratio (CNR) of the right locus coeruleus (LC), left substantia nigra (SN), the volume of left SN and the derived model combining these measures given by (2×NMV-SN-L +95×NMCNR-SN-L +28×NMCNR-LC-R).
DOI: https://doi.org/10.58530/2023/3207