3202
Sex differences in brain structure in de novo Parkinson’s disease: A cross-sectional and longitudinal neuroimaging study1Department of Radiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China, 2MR Scientific Marketing, Siemens Healthineers, Beijing, China
Synopsis
Keywords: Parkinson's Disease, Parkinson's Disease, sex difference, brain structure, cross-sectional, longitudinal
The aim of this study was to investigate sex differences in brain structure at baseline and sex differences in longitudinal changes at follow-up between female and male PD patients after excluding the expected effects of age and sex. At baseline, male PD patients exhibited significantly lower brain volume and decreased cortical thickness than age-matched female PD patients. At follow-up, these sex differences remained stable and analogous progressive brain atrophy with no sex interaction were found in male and female PD patients, suggesting similar trend in disease progression between sexes over time.Background
Parkinson’s disease (PD) varies in occurrence, presentation, and severity between the sexes1-2, suggesting that PD may involve distinct pathogenetic mechanisms in females and males. Few studies have showed that significantly smaller total cortical and subcortical grey matter volume (GMV)3, reduced cortical thickness, and lower connection strengths4 in males with PD than in females. However, corrections for brain volume, age or sex effects existing in healthy controls are rarely taken into consideration in those cross-sectional studies. Furthermore, the sex-related longitudinal changes in PD brain structure remain largely unexplored. In this work, we aimed to compare sex differences in brain features cross-sectionally and longitudinally using GMV proportion and cortical thickness in a large sample of newly diagnosed drug-naive PD patients.Materials and methods
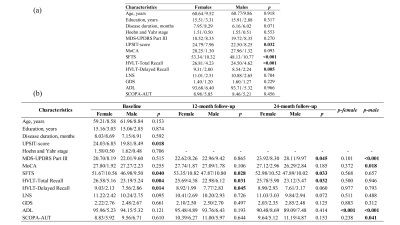
Cognitive assessments and T1-weighted MR images of 262 PD patients (171 males, defined as PD group 1) and 113 healthy controls (68 males) were selected from the Parkinson Progression Markers Initiative. Of these, 97 PD patients (66 males, defined as PD group 2) completed 12- and 24-month follow-up examinations. MR images were acquired on 1.5T or 3T MRI scanners from Philips Medical Systems, GE Medical Systems, and SIEMENS Medical Systems. The W-score approach proposed by La Joie et al.5 was used to regress out the possible normal brain gender difference and the effect of age on brain structure. At baseline, two-sample t tests were used to compare sex differences in cognitive assessments, GMV proportion and cortical thickness cross-sectionally. At follow-up, in PD group 2, mixed repeated measures analysis of variance was performed to explore the differences in longitudinal changes between males and females, with visit time as a within-group factor and sex as a between-group factor. In addition, the brain regions showing significant sex differences in PD group 1 were defined as regions of interest (ROIs) to determine the longitudinal patterns of ROIs during follow-up.Results
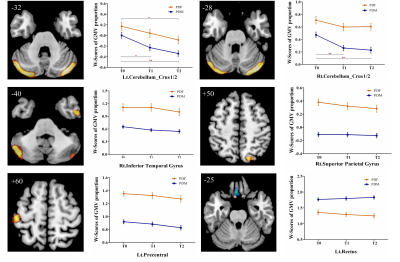
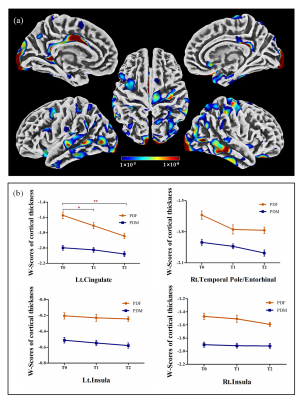
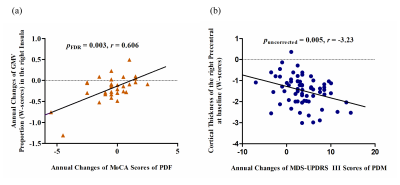
At baseline, no significant differences in age, education level, disease duration, disease stage, or disease severity were found between female and male PD patients, while males showed significantly greater olfactory dysfunction and cognitive declines in semantic fluency and memory, both in PD group 1 (Figure 1a) and PD group 2 (Figure 1b). Meanwhile, brain atrophy and cortical thickness were significantly reduced in male PD patients compared with females, mainly in the cerebellum, frontal lobe, parietal lobe, and temporal lobe (Figure 2 and Figure 3). At follow-up, in PD group 2, these sex differences remained stable, and no significant sex-related difference in longitudinal brain structural changes was observed, suggesting that disease progression dynamics are similar in female and male PD patients. Also, no significant sex-related difference in longitudinal changes of clinical assessments was found between male and female PD patients. Only the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale III (MDS-UPDRS Part III) scores showed a trend of greater longitudinal decline in males than in females (p = 0.075). Furthermore, annual changes in Montreal Cognitive Assessment (MoCA) scores in female PD patients were positively correlated with the annual changes in GMV proportion in the right insula, and the cortical thickness in the right precentral gyrus at baseline showed a tendency negatively associated with the annual change in MDS-UPDRS Part III scores in male PD patients (Figure 4).Discussion
PD is considered a movement disorder mainly related to dysfunction of motor circuit. The brain structural changes reported in the present study in the motor circuit, including the bilateral cerebellum and the precentral gyrus, may be the underlying mechanism for the more severe motor dysfunction in male PD patients. In addition, significant sex differences were found in the cingulate, insula, entorhinal cortex and some temporal and parietal brain regions, which may be associated with symptom discrepancies in cognition, language, and olfactory function6-8 between male and female PD patients. Oestrogen-related studies have showed that the beneficial effect of oestrogen use was observed in the early stage of PD in females, but not in more advanced stages9. Consistent with these, no longitudinal sex-specific change pattern was observed over time in the present study, which may be due to the disappearance of oestrogen protection effect in more advanced stage in female PD patients.Conclusions
The study demonstrated sex effect in neuroanatomy during the course of PD, provided new insights into the neurodegenerative process, and facilitated the development of more effective sex-specific therapeutic strategies in the early stage of PD.Acknowledgements
PPMI is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including Abbott, Avid, Biogen idec, Bristol-Myers Squibb, Covance, Elan, GE Healthcare, Genentech, GSK, Lilly, MERCK, MSD, Pfizer, Roche and UCB (details of the PPMI funding partners found at www.ppmiinfo.org/ fundingpartners). This work was supported by grants from the National Natural Science Foundation of China (62076169) and Beijing Hospitals Authority Youth Program (QML20200304).References
1. Iwaki H, Blauwendraat C, Leonard H L, et al. Differences in the Presentation and Progression of Parkinson's Disease by Sex[J]. Movement Disorders, 2021,36(1):106-117.
2. Haaxma C A, Bloem B R, Borm G F, et al. Gender differences in Parkinson's disease[J]. Journal of Neurology, Neurosurgery & Psychiatry, 2007,78(8):819-824.
3. Oltra J, Uribe C, Campabadal A, et al. Sex Differences in Brain and Cognition in de novo Parkinson's Disease[J]. Frontiers in Aging Neuroscience, 2022,13.
4. Yadav S K, Kathiresan N, Mohan S, et al. Gender-based analysis of cortical thickness and structural connectivity in Parkinson’s disease[J]. Journal of Neurology, 2016,263(11):2308-2318.
5. La Joie R, Perrotin A, Barre L, et al. Region-Specific Hierarchy between Atrophy, Hypometabolism, and ꞵ-Amyloid (Aꞵ) Load in Alzheimer's Disease Dementia[J]. Journal of Neuroscience, 2012,32(46):16265-16273.
6. Segura B, Baggio H C, Marti M J, et al. Cortical thinning associated with mild cognitive impairment in Parkinson's disease[J]. Movement Disorders, 2014,29(12):1495-1503.
7. Georgiopoulos C, Warntjes M, Dizdar N, et al. Olfactory Impairment in Parkinson’s Disease Studied with Diffusion Tensor and Magnetization Transfer Imaging[J]. Journal of Parkinson's Disease, 2017,7(2):301-311.
8. Jia X, Wang Z, Yang T, et al. Entorhinal Cortex Atrophy in Early, Drug-naive Parkinson’s Disease with Mild Cognitive Impairment[J]. Aging and disease, 2019,10(6):1221.
9. Strijks E, Kremer J A, Horstink M W. Effects of female sex steroids on Parkinson's disease in postmenopausal women[J]. Clin Neuropharmacol, 1999,22(2):93-97.
Figures