3200
Glymphatic system impairment in Parkinson’s disease with rapid movement sleep behavior disorder1Guangzhou First People's Hospital, Guangzhou, China, 2Guangzhou Women and Children's Medical Center, Guangzhou, China, 3The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China, 4Philips Healthcare, Guangzhou, China
Synopsis
Keywords: Parkinson's Disease, Diffusion Tensor Imaging, glymphatic system;rapid movement sleep behavior disorder
To assess the activity of the glymphatic system in PD patients with probably rapid movement sleep behavior disorder (PD-pRBD) using diffusion tensor image analysis along the perivascular space( DTI-ALPS) index. 61 PD-pRBD, 92 PD patients with no probable RBD(PD-npRBD) and 59 healthy control (HC) were included in the study. The results showed that the ALPS index in PD-pRBD group was lower than that in PD-npRBD group. The index of ALPS in PD-pRBD was negatively correlated with REM sleep behavior disorder screening questionnaire(RBDSQ) score. We concluded that the glymphatic system function of PD with RBD is impaired.Introduction
Alpha-synucleinopathy (α-syn) is postulated to be central to Parkinson’s disease (PD) with rapid eye movement sleep behavior disorder (RBD). However, the clearance mechanism of α-syn has been poorly studied. The brain’s lymphatic drainage system termed the ‘glymphatic system’, may contribute to the rapid clearance of macromolecules and protein aggregation in cerebrospinal fluid (CSF) circulation[1]. But there is insufficient evidence supporting the association between glymphatic system malfunction and α-syn in humans. Therefore, identifying a neuroimaging marker to detect glymphatic system changes in patients with PD and RBD is challenging. Recently, a technique called diffusion tensor image analysis along the perivascular space (DTI-ALPS) was introduced to assess glymphatic function without the need for a contrast agent injection, which has been demonstrated using classical glymphatic MRI and validated in several neurodegenerative disorders[2, 3]. The aim of this study was to use the DTI-ALPS to assess the activity of the glymphatic system in PD patients with probably RBD (PD-pRBD) and its relationship to clinical scores of disease severity.Methods
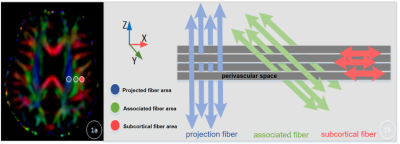
A total of 153 initially diagnosed PD patients and 59 HC were selected from the Parkinson's Progression Markers Initiative (PPMI) baseline database. According to the RBDSQ, PD patients were divided into 61 cases of PD-pRBD and 92 cases of PD-npRBD . Clinical information, MRI images, and cerebrospinal fluid biomarkers of all 212 subjects were integrated, and all MRI images were collected on a 3.0T Siemens. Diffusion tensor images were acquired to calculate diffusivity in the x, y, and z axes of the plane of the lateral ventricle body in each subject.(Fig.1) Then we evaluated the diffusivity along the perivascular spaces (PVS) as well as projection fibers and association fibers separately, to acquire an index for diffusivity along the perivascular space (ALPS-index). Differences in ALPS-index among the PD-pRBD group, PD-npRBD, and HC groups were also assessed, and further correlation analysis between the DTI-ALPS index and clinical characteristics was conducted in PD and PD-pRBD patients.Results
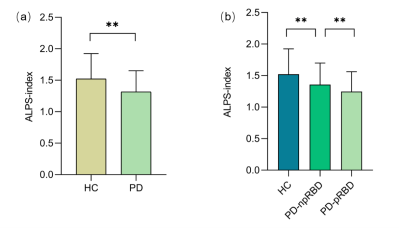
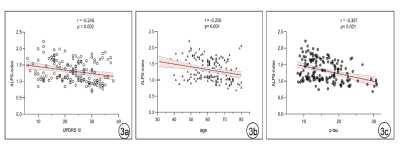
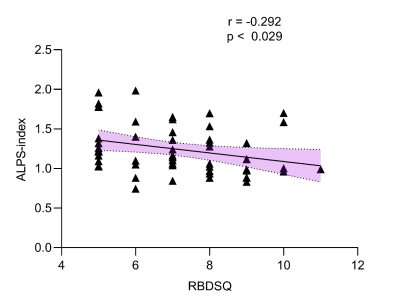
Patients with PD exhibited a lower ALPS index than HCs (Z=3.769, p<0.001), and PD-pRBD patients showed a lower ALPS index than PD-npRBD patients (Z=1.753, p<0.001). (Fig.2) The ALPS index was negatively correlated with age in the PD group (r=-0.256, p=0.001). (Fig.3) After controlling for age, gender, and years of education, the ALPS index in the PD group was negatively correlated with the Unified Parkinson’s Disease Rating Scale III score (r=-0.245, p=0.002) and the concentration of p-tau protein in cerebrospinal fluid (r=-0.357, p< 0.001); while the ALPS index was negatively correlated with RBDSQ score in the PD-pRBD group (r=-0.292, p=0.029). (Fig.4)Discussion
The results showed that the DTI-ALPS index was lower in all PD groups than in HC, with the DTI-ALPS index of PD-pRBD patients being lower than PD-npRBD patients, and the PD-npRBD group being lower than HC. Aquaporin-4 (AQP4) has been proposed to support PVS fluid and solute movement along the glymphatic system[4]. And glymphatic drainage was reduced in A53T mice, which was accompanied by impaired polarisation of AQP4 closely surrounding α-syn deposition. A previous study reported delayed glymphatic drainage in mice injected with preformed α-syn fibrils and revealed that glymphatic endothelial barrier dysfunction was induced by α-syn meningeal macrophages[5]. These experiments are consistent with our findings that glymphatic dysfunction might be closely related to α-syn accumulation. Our results showed that the DTI-ALPS index was negatively correlated with age, URDRS III score, and p-tau protein concentration in cerebrospinal fluid (CSF) in the PD group. Studies have shown that hypertension and loss of AQP-4 may affect local interstitial fluid drainage and destroy α-synuclein clearance during brain aging, among which PVS is an important pathway[6]. MDS-UPDRS III score reflects the severity of PD, suggesting that the glymphatic system function may further decrease with the progression of PD[7]. Tau is a microtubule-associated protein that may be released into the extracellular space in some neurodegenerative diseases. α-synuclein can accelerate the phosphorylation of tau (p-tau), which can disrupt axonal transport and lead to further aggregation of α-synuclein in cells[8]. In our results, the ALPS index was inversely associated with RBD disease severity. It has been documented that the lymphoid system can expel ISF and metabolic waste products more efficiently during sleep[9]. Thus, sleep disorders have the potential to disrupt waste clearance mechanisms through the glymphatic system and may promote the accumulation of toxic proteins. Therefore, sleep-related disorders in RBD may alter these clearance mechanisms. We hypothesized that in patients with PD-pRBD, sleep disruption and neuroinflammation during REM may lead to increased metabolic deposition and a-synuclein aggregation, and abnormal a-synuclein aggregation may further hinder the function of the glymphatic system.Conclusions
DTI-APLS index can be used to evaluate the impairment of lymphoid system function in PD-pRBD patients, which provides a new idea for exploring the pathogenesis of PD with RBD.Acknowledgements
No acknowledgement found.References
[1] Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018. 17(11): 1016-1024.
[2] Ma X, Li S, Li C, et al. Diffusion Tensor Imaging Along the Perivascular Space Index in Different Stages of Parkinson's Disease. Front Aging Neurosci. 2021. 13: 773951.
[3] Du G, Lewis MM, Kanekar S, et al. Combined Diffusion Tensor Imaging and Apparent Transverse Relaxation Rate Differentiate Parkinson Disease and Atypical Parkinsonism. AJNR Am J Neuroradiol. 2017. 38(5): 966-972.
[4] Chen H, Wan H, Zhang M, Wardlaw JM, Feng T, Wang Y. Perivascular space in Parkinson's disease: Association with CSF amyloid/tau and cognitive decline. Parkinsonism Relat Disord. 2022. 95: 70-76.
[5] Donahue EK, Murdos A, Jakowec MW, et al. Global and Regional Changes in Perivascular Space in Idiopathic and Familial Parkinson's Disease. Mov Disord. 2021. 36(5): 1126-1136.
[6] Si X, Guo T, Wang Z, et al. Neuroimaging evidence of glymphatic system dysfunction in possible REM sleep behavior disorder and Parkinson's disease. NPJ Parkinsons Dis. 2022. 8(1): 54.
[7] Hablitz LM, Nedergaard M. The glymphatic system. Curr Biol. 2021. 31(20): R1371-R1375.
[8] Busche MA, Hyman BT. Synergy between amyloid-β and tau in Alzheimer's disease. Nat Neurosci. 2020. 23(10): 1183-1193.
[9] Benveniste H, Heerdt PM, Fontes M, Rothman DL, Volkow ND. Glymphatic System Function in Relation to Anesthesia and Sleep States. Anesth Analg. 2019. 128(4): 747-758.
Figures