3198
Baseline cerebral structural morphology predicts future freezing of gait in early drug-naïve Parkinson’s disease1The Second Affiliated Hospital, School of Medicine, South China University of Technology, Guangzhou, China, 2Affiliated Dongguan Hospital, Southern Medical University (Dongguan People's Hospital), Dongguan, China, 3Philips Healthcare, Guangzhou, China, 4Guangzhou First People's Hospital, Guangzhou, China
Synopsis
Keywords: Parkinson's Disease, Parkinson's Disease, drug-naïve Parkinson’s disease, Freezing of gait (FOG)
We developed a model that could predict the occurrence of Freezing of gait (FOG) at the individual level using machine learning with the clinical, laboratory, and cerebral structural imaging information of early drug-naïve Parkinson’s disease (PD) patients. Data from 158 early drug-naïve PD patients at baseline were obtained from the Parkinson’s Progression Markers Initiative cohort. The predictive performance of future FOG in early PD was evaluated using elastic net-support vector machine models. T1WI morphometric markers have the potential to help predict future FOG in patients with early PD at an individual level, with improved performance when integrated with clinical variables.Introduction
FOG could significantly weaken the ability of motion and diminish the quality of life in PD patients.1,2 Predictors of FOG in early PD are limited. Therefore, we aimed to use machine learning combined with clinical, laboratory, and structural brain imaging data from patients with early drug-naïve PD to develop a model that can predict the onset of FOG at the individual level.Methods
This study's baseline data of 158 early drug-naïve PD patients came from the Parkinson's Disease Progression Marker Initiative cohort. Incident FOG behavior was defined as a positive score on the Questionnaire for the Unified Parkinson’s Disease Rating Scale items 2.13 and 3.11. The CIVET pipeline was used to generate structural morphological features with T1WI, including cortical thickness, surface area, surface mean curvature, gray matter volumes, and white matter volumes. The elastic net-support vector machine model was used to assess the predictive performance of future FOG in early PD during the five-year follow-up period.Results
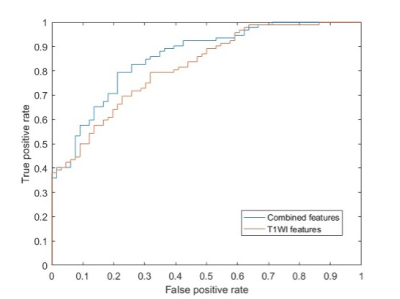
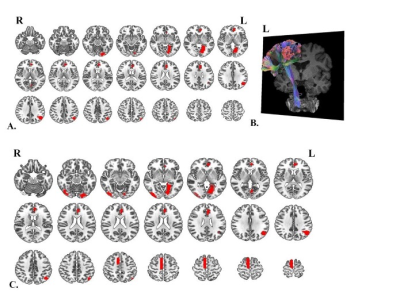
Sixty-six (41.8%) patients with PD developed FOG during the follow-up period. The median time to the first occurrence of FOG was 29 (2-96) months from baseline. The model trained with structural morphological features showed fair to good performance (AUC 0.73; accuracy 0.72) (Fig.1). Performance improved when the model was trained with structural morphological features and clinical and laboratory variables (AUC 0.77; accuracy 0.78) (Fig.1). The main features used to predict FOG at baseline in PD patients based on elastic net-support vector machine models were the left lingual gyrus, left anterior cingulate and paracingulate gyri and left angular gyrus (Fig.2).Discussion
The model developed in this study using structural features with and without clinical and laboratory features performed better than the previous study.3-5 We found that several disrupted brain regions that might help predict future FOG were mainly distributed in the occipital lobe, limbic systems, and part of the frontoparietal lobes. Decreasing grey matter volume in the left lingual and angular gyrus might lead to visual disturbances and even visual hallucinations. When patients with PD suffer from visual disturbances and/or hallucinations, they are more likely to fall and have worse cognitive and executive function, and there is greater fear of falling, leading to the possible development of FOG.6,7Conclusion
Our findings demonstrated the potential of T1WI morphometric markers, which include areas of the limbic system, occipital lobes, and frontal lobes, to aid in predicting future FOG in patients with early PD and these markers may have higher predictive performance when combined with clinical investigations.Acknowledgements
This study was supported by the Natural Science Foundation of Guangdong Province (2021A1515011288), the Science and Technology Project of Guangzhou (202102010020), and the Special Clinical Technology of Guangzhou (2019TS46).References
1. Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 2011;10(8):734-744.
2. Canning CG, Paul SS, Nieuwboer A. Prevention of falls in Parkinson's disease: a review of fall risk factors and the role of physical interventions. Neurodegener Dis Manag. 2014;4(3):203-21. doi:10.2217/nmt.14.22
3. Xu K, Zhou XX, He RC, et al. Constructing Prediction Models for Freezing of Gait by Nomogram and Machine Learning: A Longitudinal Study. Front Neurol. 2021;12:684044.
4. Kim R, Lee J, Kim Y, et al. Presynaptic striatal dopaminergic depletion predicts the later development of freezing of gait in de novo Parkinson's disease: An analysis of the PPMI cohort. Parkinsonism Relat Disord 2018;51:49-54.
5. Kim R, Lee J, Kim HJ, et al. CSF beta-amyloid42 and risk of freezing of gait in early Parkinson disease. Neurology 2019;92(1):e40-e47.
6. Heremans E, Nieuwboer A, Vercruysse S. Freezing of gait in Parkinson's disease: where are we now? Curr Neurol Neurosci Rep. Jun 2013;13(6):350.
7. Lord SR, Bindels H, Ketheeswaran M, et al. Freezing of Gait in People with Parkinson's Disease: Nature, Occurrence, and Risk Factors. J Parkinsons Dis.
Figures