3197
Association between subregions of the substantia nigra and behavioral symptoms in patients with Parkinson disease using neuromelanin MRI1Neurology, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Keywords: Parkinson's Disease, Neuroscience
We investigated the relationship between subregions of the substantia nigra and behavioral disinhibition in patients with Parkinson disease using neuromelanin MRI. We found a significant positive relationship between disinhibition and neuromelanin MRI contrast ratio in bi-lateral clusters localized in the medial substantia nigra. These findings suggest that neuromelanin MRI can be used to assess the localization of changes in the substantia nigra and inform how dopamine pathophysiology relates to behavioral symptoms in Parkinson disease.Introduction
In Parkinson disease (PD), degeneration of dopaminergic neurons alter nigrostriatal and mesolimbic networks leading to motor and non-motor symptoms 1,2. Impulsivity is a non-motor manifestation of PD, and increased disinhibition is described as a symptom of PD 3,4. Dopamine networks converge in the midbrain 5, and recent studies suggest a somatotopy of the substantia nigra (SN) 5–8. This somatotopy parcellates the SN medial-limbic, ventral-executive, and lateral-motor networks. As such, in vivo measurements that can assess the subregions of the SN 6,9,10 could inform how dopamine pathophysiology relates to behavior 11–13. Neuromelanin sensitive MRI (NM-MRI) 14 allows the visualization of the SN, and the contrast ratio (CR) of NM-MRI signal in the SN to a reference region in the adjacent cerebral crus (CC) is a proposed assay of nigral integrity 15. The overall goal of this work was to apply submillimeter magnetization transfer-weighted NM-MRI at the spatial resolution of the SN to test fundamental hypotheses regarding NM contrast and clinical indicators of disinhibition in patients with PD. We tested the hypothesis that preserved NM-MRI signal in the medial subregion of the SN reflects a more intact limbic dopamine network and is the basis for disinhibition in a cohort of PD patients.Methods
Patients with a clinical diagnosis of PD were recruited prospectively from the neurological services at our hospital and provided informed, written consent. The Frontal Systems Behavioral Scale (FrSBe) 16 was completed by the caregiver of all participants, and the disinhibition sub-score was used to assess the severity of behavioral disinhibition.All participants underwent brain MRI on a 3T scanner (Philips) with a 32-channel phased-array reception including a 3D T1-weighted scan, (MPRAGE, TR/TE=8.9/4.6 ms; turbo-gradient-echo factor=131; spatial resolution=1x1x1 mm3), and NM-MRI consisting of a T1-weighted turbo spin echo (TSE) sequence with off-resonance magnetization transfer preparation (TR/TE = 670/12 ms, in-plane spatial resolution = 0.6×0.6 mm2, slice thickness = 2.5 mm, signal averages = 5, duration=5 min 55 s). For the NM-MRI acquisition, the field-of-view was placed perpendicular to the floor of the fourth ventricle.
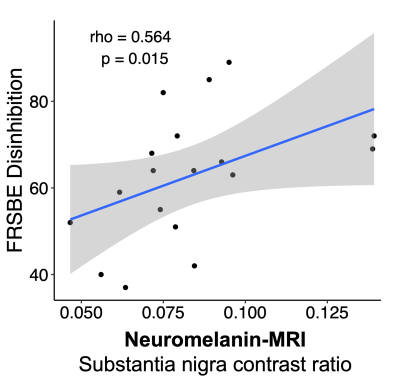
NM-MRI scans were initially analyzed in subject space and global SN neuronal integrity was assessed by measuring the contrast ratio between the signal intensity in the visible SN relative to a background reference region in the adjacent CC. The region of interest for the SN and CC was manually segmented for each subject, and the contrast ratio was calculated as (CR=(ICC-ISN)/ ICC), where ICC and ISN are the mean signal intensity in the CC and SN, respectively). The Spearman correlation coefficient was used to test the relationship between the NM-MRI SN contrast ratio and the FrsBe disinhibition score.
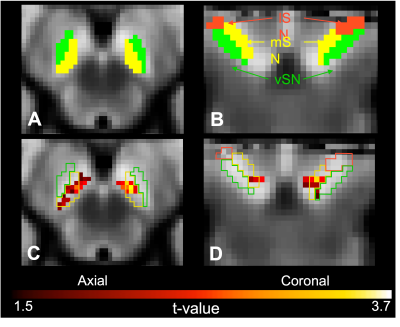
Subsequently, we investigated the subregion-specific relationship between SN contrast and disinhibition by using a voxel-wise analysis 15. NM-MRI images were coregistered to the T1-weighted images using rigid registration and subsequently non-lineally transformed into the MNI T1-weighted template using Advanced Normalization Tools (ANTs). A template NM-MRI image was created by averaging the spatially normalized NM-MRI images from all participants, and the hyperintense SN-ventral tegmental area (VTA) complex and hypointense CC reference region were manually traced on this NM-MRI template. The contrast-ratio maps were obtained by calculating the CR for each voxel in the SN-VTA using the same CR formula as above where ICC is the mean signal of the CC, and ISN is the signal at each voxel of the SN-VTA. To evaluate the location-specific relationship between SN integrity and disinhibition, a voxel-wise analysis was performed using the Matlab SurfStat toolbox 17. Multi-linear regression was used to assess the relationship between the CR as independent variable, with FrSBe disinhibition score as dependent variable, with age and sex as covariates. Lastly, we used the SN atlas 6 to understand if the clusters obtained from the voxel-wise analysis corresponded to a somatotopy parcellation of the SN.
Results
Eighteen PD patients (age=64.1±5.4 years; 13 males; disease duration=5.1±2.9 years; UPDRS-II=12.6±7.6; UPDRS-III=31.1±13.4) were enrolled. We found a significant positive relationship between global SN CR and FrSBe disinhibition score (rho=0.564, p-value=0.015) (Figure 1). The voxel-wise analysis in the SN using multi-linear regression model showed a significant relationship between NM-MRI signal and FrSBE disinhibition score (Figure 2). Findings were localized in bi-lateral clusters of voxels in the medial SN and extending into the VTA (p-values for cluster size<0.001, peak p-value=0.0014).Discussion
Given the anatomical and functional heterogeneity of the human dopamine neurons in the SN, it is important to investigate regional changes along the SN and how these changes relate to motor and non-motor symptoms in PD. In vivo measurements that can assess the subregions of the SN are critical for understanding how changes in the different dopamine networks can impact the presentation of impulsivity. The results of this study leverage unique contrast provided by magnetization transfer-weighted NM-MRI at high spatial resolution to provide insights into non-motor correlates of the medial-SN subregion in patients with PD and could be used to inform how pathophysiology in the limbic dopamine network relates to impulsive behaviors.Conclusion
Neuromelanin MRI can be used to assess localization of SN pathology related to behavioral symptoms in PD, and may provide a useful non-invasive biomarker of nigral dopamine integrity in PD.Acknowledgements
This study was supported by the NIH/NINDS R01 NS097783 and K23 NS080988.References
1. Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience. 2014;282:248-257.
2. Ikemoto S, Satoshi Ikemoto, Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56(1):27-78.
3. Voon V, Hassan K, Zurowski M, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67(7):1254-1257.
4. Bostwick JM, Hecksel KA, Stevens SR, Bower JH, Ahlskog JE. Frequency of new-onset pathologic compulsive gambling or hypersexuality after drug treatment of idiopathic Parkinson disease. Mayo Clin Proc. 2009;84(4):310-316.
5. Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4-26.
6. Zhang Y, Larcher KMH, Misic B, Dagher A. Anatomical and functional organization of the human substantia Nigra and its connections. Elife. 2017;6.
7. Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459(7248):837-841.
8. Nomoto K, Schultz W, Watanabe T, Sakagami M. Temporally extended dopamine responses to perceptually demanding reward-predictive stimuli. Journal of Neuroscience. 2010;30(32):10692-10702.
9. Biondetti E, Gaurav R, Yahia-Cherif L, et al. Spatiotemporal changes in substantia nigra neuromelanin content in Parkinson’s disease. Brain. 2020;143(9):2757-2770.
10. Vaillancourt DE, Mitchell T. Parkinson’s disease progression in the substantia Nigra: Location, location, location. Brain. 2020;143(9):2628-2630.
11. Dagher A, Robbins TW. Personality, Addiction, Dopamine: Insights from Parkinson’s Disease. Neuron. 2009;61(4):502-510.
12. Dalley JW, Robbins TW. Fractionating impulsivity: Neuropsychiatric implications. Nat Rev Neurosci. 2017;18(3):158-171.
13. Morris LS, Voon V. Dimensionality of Cognitions in Behavioral Addiction. Curr Behav Neurosci Rep. 2016;3(1):49-57.
14. Sulzer D, Cassidy C, Horga G, et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. NPJ Parkinsons Dis. 2018;4(1):11.
15. Cassidy CM, Zucca FA, Girgis RR, et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proceedings of the National Academy of Sciences. 2019;116(11):5108-5117.
16. Stout JC, Ready RE, Grace J, Malloy PF, Paulsen JS. Factor Analysis of the Frontal Systems Behavior Scale (FrSBe) Assessment. 2003 Mar;10(1):79-8.
17. Worsley K, Taylor J, Carbonell F, et al. SurfStat: A Matlab toolbox for the statistical analysis of univariate and multivariate surface and volumetric data using linear mixed effects models and random field theory. Neuroimage. 2009;47:S102.
Figures