3196
Altered Hippocampal Subregions Volumes Is Associated With Emotional Impairment of Parkinson’s Disease1The First Affiliated Hospital of Dalian Medical University, Dalian, China, 2GE Healthcare, Shanghai, China
Synopsis
Keywords: Parkinson's Disease, Psychiatric Disorders
Depression is the main emotional manifestation associated with Parkinson's disease. The hippocampus is a core region in the limbic system, critical in regulating emotion. This study explored the subregional atrophy pattern of the hippocampus in Parkinson's disease with depression (DPD) patients and its correlation with the severity of depressive symptoms. The results of this study suggest that DPD patients exhibit a left asymmetric atrophy pattern in the hippocampus and its subregions. Partial correlation analysis showed that the left dentate gyrus, left molecular layer, left CA4 and left hippocampal volumes were negatively correlated with Hamilton depression scale scores in DPD patients.Summary of Main Findings
This study explored the subregional atrophy pattern of the hippocampus in Parkinson's disease with depression (DPD) patients and its correlation with the severity of depressive symptoms. The results of this study suggest that DPD patients exhibit a left asymmetric atrophy pattern in the hippocampus and its subregions, especially in the left molecular layer, left dentate gyrus, left CA3, and left CA4. Partial correlation analysis showed that the left hippocampal dentate gyrus, left hippocampal molecular layer, left hippocampal CA4 and left hippocampal volumes were negatively correlated with Hamilton depression scale scores in DPD patients.Introduction
Depression affects 40%–50% of Parkinson's disease (PD) patients, is the most important factor impairing patients' quality of life, and is frequently unrecognized and undertreated[1]. The hippocampus is closely related to the pathogenesis of DPD. The hippocampus is a highly stress-sensitive brain region. Chronic stress and hypersecretion of cortisol can exert neurotoxic effects on the hippocampus, leading to impaired hippocampal structure[2]. A study has found the relationship between hippocampus volume and depressive symptoms in PD[3]. However, there are almost no studies on changes in hippocampal subregion volumes in DPD patients. In this study, we aim to use an automatic hippocampal segmentation method to determine if there was a change in subregion hippocampal volume in DPD patients.Materials and Methods
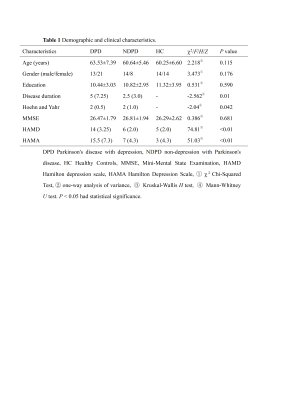
This study included 56 patients with PD (34 in the DPD group, 22 in the NDPD group) and 28 healthy controls (HC) matched by sex, age and education. We used the Hamilton Depression Scale (HAMD), with a HAMD score of >7 in the DPD group and a HAMD score of ≤7 in the non-depression with Parkinson's disease (NDPD) group. All Participants were scanned using a 3.0 T GE Signa HDXT scanner from America equipped with an 8-channel head coil. A 3D magnetization-prepared rapid-acquisition gradient-echo T1-weighted sequence with the following parameters was performed: repetition time = 10.2 ms, echo time (shortest) = 4.2 ms, flip angle = 13°, FOV = 24 × 24cm2, matrix size = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm, slice thickness 1.0 mm. Segmentation of hippocampal subregions was performed using FreeSurfer 6.0 software. The hippocampus was divided into 12 subregions: parasubiculum, presubiculum, subiculum, CA1, CA3, CA4, granule cell layer of the dentate gyrus (GC-DG), hippocampus-amygdala transition area (HATA), fimbria, molecular layer, hippocampal fissure, and hippocampal tail. The estimated total intracranial volume (eTIV) volumes were also extracted. Statistical analyses were performed using SPSS 26.0 software. Covariance analysis (ANOVA) was applied to compare the hippocampal subregion volume differences among the three groups. Partial correlation analysis was performed to evaluate the correlation between significantly reduced hippocampal subregion volumes and the severity of depressive symptoms. Age, gender, education, and eTIV were included as covariates. The FDR was used for correction.Result
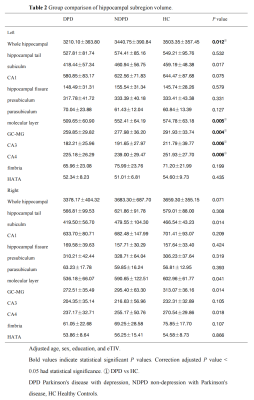
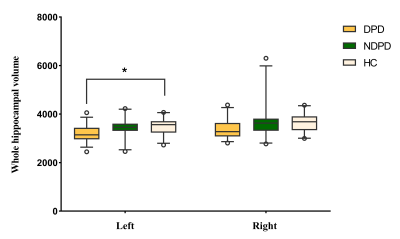
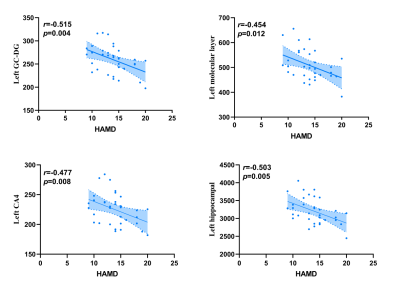
The demographic information and clinical data are summarized in Table 1. As shown in Fig. 1. We found that the left total hippocampal volume was significantly reduced in the DPD group compared with the HC group (P = 0.012). Further comparison of bilateral hippocampal subregions volumes revealed the differences between the DPD group and the HC group in the left molecular layer (P = 0.004), left GC-DG (P = 0.003), left CA3 (P = 0.007), and left CA4 (P = 0.005) subregion volumes remained statistically significant. However, the differences in the right hippocampal subregion volumes were no longer significantly different after correction for multiple comparisons. (Table 2, Fig. 2). In the DPD group, partial correlation analysis showed that the left GC-DG (r = -0.515, P = 0.004), left molecular layer (r = -0.454, P = 0.012), left CA4 (r = -0.477, P = 0.008) and left hippocampal volumes (r = -0.503, P = 0.005) were negatively correlated with HAMD scores (Fig. 3).Discussion
In this study, we found that Different hippocampal subregions may be impaired differently in the pathogenesis of DPD. The results of this study suggest that DPD patients exhibit an asymmetric atrophy pattern in the hippocampus and its subregions, mainly in the left hippocampus and its subregions. Previous studies have found asymmetric atrophy of left hippocampal atrophy in patients with major depressive disorder[4]. The CA3, CA4 and GC-DG subregions are components of the classical trisynaptic circuit. Disrupting any of these subregions would alter circuit function, thus altering sensory and emotional information processing and producing depressive symptoms[5]. Preclinical studies suggest that dendritic retraction in CA3-4 are more sensitive to chronic psychological stress and that antidepressants can prevent or reverse stress effects[6]. The molecular layer is enriched with nerve fibres that transmit sensory information from the internal olfactory cortex, which may be critical in integrating aspects of emotional information.Conclusion
This study found that DPD patients exhibit a left asymmetric atrophy pattern in the hippocampus and its subregions, especially in the left molecular layer, left dentate gyrus, left CA3, and left CA4. Partial correlation analysis showed that the left hippocampal dentate gyrus, left hippocampal molecular layer, left hippocampal CA4 and left hippocampal volumes were negatively correlated with Hamilton depression scale scores in DPD patients.Acknowledgements
No acknowledgement found.References
1. Taylor AE, Saint-Cyr JA, Lang AE, Kenny FT. Parkinson's disease and depression. A critical re-evaluation. Brain. 1986 Apr;109 ( Pt 2):279-92.
2. Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci. 2007 Mar 14;27(11):2734-43.
3. van Mierlo TJ, Chung C, Foncke EM, Berendse HW, van den Heuvel OA. Depressive symptoms in Parkinson's disease are related to decreased hippocampus and amygdala volume. Mov Disord. 2015 Feb;30(2):245-52.
4. Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jäger M, Leinsinger G, Bottlender R, Hahn K, Möller HJ. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry. 2002 Jul;159(7):1112-8.
5. Stepan J, Dine J, Eder M. Functional optical probing of the hippocampal trisynaptic circuit in vitro: network dynamics, filter properties, and polysynaptic induction of CA1 LTP. Front Neurosci. 2015 May 6;9:160.
6. Czéh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007 Aug;257(5):250-60.
Figures