3189
Visualization of Human Neurofluids Using Intrathecal 17O-Labeled Water and MRI: A Preliminary Study1Department of Diagnostic and Interventional Radiology, Hokkaido University Hospital, Sapporo, Japan, 2Department of Raiology, Faculty of Dental Medicine, Hokkaido University, Sapporo, Japan, 3Department of Diagnostic Imaging, Facaulty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Japan, 4Faculty of Health Sciences, Hokkaido University, Sapporo, Japan, 5Global Center for Biomedical Science and Engineering, Hokkaido University, Sapporo, Japan
Synopsis
Keywords: Neurofluids, Neurofluids
Imaging of neurofluids dynamics is a promising aspect in clinical imaging. Only few methods of long-term water tracking in the brain have been described for diagnostic purposes. Herein, we examined water circulation between the CSF and ISF using an intrathecal injection of 17O-labeled water and 3T-MRI in two patients with dementia. Signal changes were detected in the brain parenchyma after the intrathecal administration. This visualization technique can detect the distribution of water tracers to the CSF and brain parenchyma and could become a clinical tool for evaluating neurofluids dynamics in humans.INTRODUCTION
The neurofluids acts as the clearance system of the brain through circulation of the cerebral spinal fluid (CSF) and cerebral interstitial fluid (ISF). Evidence of its existence has been reported in many animal and human studies1,2. Visualization of this aspect of brain physiology is expected to have a significant impact on clinical imaging. Former MRI tracer studies have used gadolinium contrast media as a soluble tracer for intrathecal injection3. However, it is associated with side effects and may not accurately reflect the dynamics of neurofluids since solutes and water molecules behave differently. 17O-labeled water, which is water labeled with a stable oxygen isotope, is a T2-shortening contrast agent in proton MRI, allowing direct imaging of water4-7. We previously reported the feasibility of water clearance assessment in the CSF using this tracer8. However, the study did not successfully evaluate the distribution of 17O in the brain parenchyma because the long echo time (TE) to target the CSF reduced the signal-to-noise ratio (SNR) of the brain parenchyma. In this study, we evaluated the visualization of water circulation between CSF and ISF in humans using an intrathecal injection of 17O-labeled water and MRI in a small number of patients.MATERIALS AND METHODS
The institutional review board approved the study, and the written informed consent was obtained from the patient. Because of the procedure’s invasiveness, only patients already scheduled for lumbar puncture were included. Two patients with suspected dementia (patient 1, an 84-year-old woman; patient 2, a 63-year-old man) were examined using MRI. After lumbar puncture examination, 10 ml of 17O-labeled physiological saline (enriched to 10 mol%, PSO17, Taiyo Nippon Sanso, Tokyo, Japan) was slowly administered intrathecally. MRI scans were performed using a 3.0 Tesla MRI scanner (Hitachi, Ltd., Tokyo, Japan). Whole-brain 3D-T2-weighted images were acquired using a fast spin echo (FSE) sequence (TR = 3500 ms; effective TE = 100 ms; FA = 90o; NSA = 1; echo train length = 35; acceleration factor = 2; matrix size = 192×192; field of view (FOV) = 320 mm; slice thickness = 1.7 mm) at four time points (baseline and post-1, 8, and 24 h; Fig. 1). Patient-specific fixation pillows were used to align the patient positions for each MRI scan. The receiver amplifier gain for each session was fixed. Statistical Parametric Mapping software (SPM12) and MATLAB R2022a was used for image alignment, segmentation, and calculation of 17O concentration maps. Changes in the 17O concentration were calculated considering the baseline signal using a previously reported formula4-6.RESULTS
The patients did not experience any adverse events after receiving 17O labeled water. In both patients, the 17O concentrations peaked in the CSF space, mainly in the basilar cistern and bilateral sylvian valley, 1 h after administration, and then declined (Fig. 2). A similar trend was observed in the brain parenchyma, but the heterogeneity of 17O was noticeable across regions. Lumbar puncture was performed in the lateral position, and the signal change was particularly pronounced in the area that was in the direction of gravity in the lateral position (Fig. 2).DISCUSSION
In this study, we detected signal changes in the brain parenchyma using clinical MRI after intrathecal administration of 17O-labelled water. We speculate that these signal changes indicate the circulation of the water tracer from the CSF space to the brain parenchyma via the perivascular space, i.e., the glymphatic pathway. Although previous studies in rats have used high 17O concentration water (90 mol%) and ultra-high field 7T MRI7, our preliminary data indicate that even a 10% concentration of 17O-labeled water and a 3T-MRI can be valuable tools for visualizing neurofluids dynamics in humans. However, our study has some limitations. First, we used weighted MR images to measure 17O signal changes; these may be influenced by session-to-session differences in the patient’s geometric position relative to the MR coil that affect the B1 profiles, and other related factors. Our next plan consists of using high-resolution 3D-T2 mapping for improving the accuracy and reliability of 17O measurements. Second, since part of the observed 17O distribution to the brain parenchyma seemed to be related to the body position at the time of injection, adjusting the body position during or after injection may be necessary to avoid uneven distribution of tracer. Third, the effects from blood circulation are unknown because 17O pharmacokinetics were not assessed by blood or spinal fluid sampling. Finally, the small number of patients presents a limitation.CONCLUSION
Direct imaging of water tracers using MRI with intrathecal administration of 17O-labeled water can detect their distribution to the CSF and brain parenchyma. This technique can become a successful clinical tool for the evaluation of neurofluids dynamics in humans.Acknowledgements
No acknowledgement found.References
- Benjamin A Plog and Maiken Nedergaard. 2018. “The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future.” Annual Review of Pathology 13: 379–94.
- Martin Kaag Rasmussen, Humberto Mestre, and Maiken Nedergaard. 2018. “The glymphatic pathway in neurological disorders.” Lancet Neurology 17 (11): 1016–24.
- Geir Ringstad, Lars M Valnes, Anders M Dale, Are H Pripp, Svein-Are S Vatnehol, Kyrre E Emblem, Kent-Andre Mardal, and Per K Eide. 2018. “Brain-wide glymphatic enhancement and clearance in humans assessed with MRI.” JCI Insight 3 (13): e121537.
- Kohsuke Kudo, Taisuke Harada, Hiroyuki Kameda, Ikuko Uwano, Fumio Yamashita, Satomi Higuchi, Kunihiro Yoshioka, and Makoto Sasaki. 2018. “Indirect MRI of 17O-labeled water using steady-state sequences: Signal simulation and preclinical experiment.” Journal of Magnetic Resonance Imaging: JMRI 47 (5): 1373–79.
- Kohsuke Kudo, Taisuke Harada, Hiroyuki Kameda, Ikuko Uwano, Fumio Yamashita, Satomi Higuchi, Kunihiro Yoshioka, and Makoto Sasaki. 2018. “Indirect Proton MR Imaging and Kinetic Analysis of 17O-Labeled Water Tracer in the Brain.” Magnetic Resonance in Medical Sciences: MRMS: an official journal of Japan Society of Magnetic Resonance in Medicine 17 (3): 223–30.
- Taisuke Harada, Kohsuke Kudo, Hiroyuki Kameda, Ryota Sato, Toru Shirai, Yoshitaka Bito, Noriyuki Fujima, et al. 2022. “Phase I Randomized Trial of 17O-Labeled Water: Safety and Feasibility Study of Indirect Proton MRI for the Evaluation of Cerebral Water Dynamics.” Journal of Magnetic Resonance Imaging: JMRI.
- Mohammed S Alshuhri, Lindsay Gallagher, Lorraine M Work, and William M Holmes. 2021. “Direct imaging of glymphatic transport using H217O MRI.” JCI Insight 6 (10): e141159.
- Hiroyuki Sugimori, Hiroyuki Kameda, Taisuke Harada, Kinya Ishizaka, Masayoshi Kajiyama, Tasuku Kimura, Niki Udo, et al. 2022. “Quantitative magnetic resonance imaging for evaluating of the cerebrospinal fluid kinetics with 17O-labeled water tracer: A preliminary report.” Magnetic Resonance Imaging 87: 77–85.
Figures
Figure 1. MRI scan schedule.
MRI data were acquired using 3D T2-weighted images (WI) at four time points: baseline and 1, 8, and 24 h after intrathecal administration of 17O labeled physiological saline.
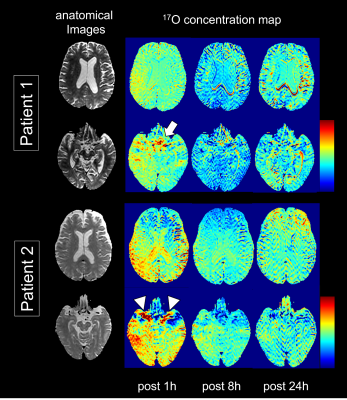
Figure 2. Brain distribution of intrathecally administered 17O water.
The color images show 17O brain distribution for each session of the evaluated patients. Both patients showed a peak 17O concentration in the CSF space 1 h after administration. The distribution of 17O was particularly high in the basilar cistern (white arrow) and bilateral Sylvian valleys (white arrowhead). After that, its concentration decreased. A similar trend is seen in the brain parenchyma but the distribution across regions is heterogeneous.