3186
Abnormal glymphatic system function in patients with chronic migraine using diffusion tensor image analysis along the perivascular space1Department of Radiology, Beijing Tiantan hospital, Capital Medical University, Beijing, China, 2Tiantan Neuroimaging Center of Excellence, China National Clinical Research Center for Neurological Diseases, Beijing, China, 3Headache Center, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China, 4Department of Neurology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 5Department of Physical Medicine and Rehabilitation of the Affiliated Sir Run Shumen Shaw Hospital and Interdisciplinary Institute of Neuroscience and Technology, School of Medicine, Zhejiang University, Hangzhou, China, 6key Laboratory of Biomedical Engineering of Education Ministry, College of Biomedical Engineering and Instrument Science, Zhejiang University, Hangzhou, China, 7MOE Frontier Science Center for Brain Science and Brain-machine Integration, School of Brain Science and Brain Medicine, Zhejiang University, Hangzhou, China
Synopsis
Keywords: Neurofluids, Diffusion Tensor Imaging, Headache
Chronic migraine (CM) is a common and highly disabling primary headache associated with vasomotion dysfunction. It is unclear whether glymphatic dysfunction develops due to frequent and chronic headache attacks in CM. In the current study, we evaluated the glymphatic system function in patients with CM using diffusion tensor image analysis along the perivascular space (DTI-ALPS) technique. We found a significantly higher ALPS index compared with healthy controls, and the alteration was significant only observed in the right hemisphere. Patients with CM have abnormalities in glymphatic function, and the right-prominent alterations might be a certain feature of CM.Introduction
Chronic migraine (CM) is a common primary headache and a very disabling disease affecting approximately 1.4 to 2.2% of the general population1. The glymphatic function of headache disorders has gradually been noticed. The glymphatic system is defined as a glial-dependent perivascular network that removes metabolic waste from the brain parenchyma2. An experimental study revealed the neuronal event accompanied by migraine aura induced closure of perivascular space and impaired the glymphatic flow3, suggesting for the first time a link between headache and glymphatic function. The bidirectional relationship between sleep and headache, that sleep disorder is a trigger for headaches while sleeping can also relieve headache attacks, indicated that the glymphatic system that is activated during sleep may play a mediated role in the headache4,5. A recent study has first found no significant abnormalities in glymphatic function in patients with episodic migraine6, but it is unclear whether glymphatic dysfunction develops due to frequent and chronic headache attacks in CM. The diffusion tensor image analysis along the perivascular space (DTI-ALPS) index measures the water diffusivity along the perivascular space by a non-invasive approach to evaluate the glymphatic activity in vivo7. Therefore, we evaluated the alterations in glymphatic system function using the ALPS index in patients diagnosed with CM compared with healthy controls.Methods
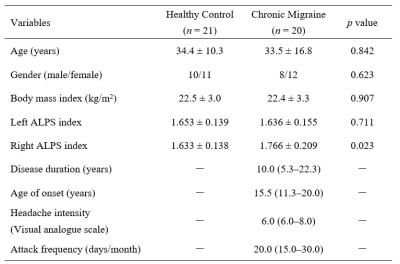
Study participantsThis study was approved by the local ethics committee, and all subjects provided written informed consent. A total of 20 patients diagnosed with CM and 21 healthy controls matched in age and sex were included in this study (Table 1).
MRI acquisition
All participants were subjected to a 3.0T MRI scanner (SIGNA Premier, GE Healthcare, USA). The following scans were performed: 1) MPRAGE (slice thickness=1mm, TR/TE=400ms/3ms); 2) T2 weighted image (slice thickness=5mm, TR/TE = 4377ms/102ms); 3) Diffusion tensor image (DTI) (slice thickness=2mm, TR/TE= 5258ms/85ms, gradient direction = 108, diffusion sensitivity coefficient (b) = 0, 1000 s/mm2). The acquisition time was 14 minutes and 40 seconds.
ALPS index calculation
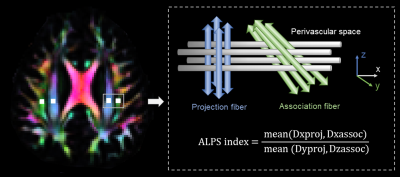
DTI-ALPS processing was performed according to the methods described in the previous publications7,8. Fractional anisotropy (FA) and color-coded FA maps were processed after preprocessing DTI data9,10. The locations of the ROIs on the projection and association fibers were determined in standard space and then transferred to individual space for each participant. Then x-, y-, and z-axes diffusivities of ROIs were recorded and the ALPS index was calculated by the formula as follows7: ALPS index=mean(Dxproj, Dxassoc)/mean(Dyproj, Dzassoc). (Figure 1)
Statistical analysis
Two sample t-tests were performed to examine the group difference in the ALPS index and paired sample t-tests were used to compare the difference in their left and right ALPS index. P < 0.05 was considered statistically significant.
Results
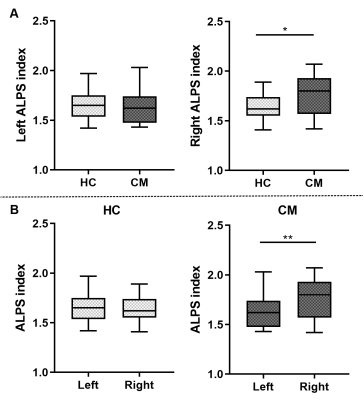
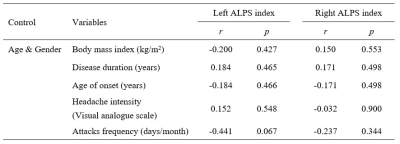
The right ALPS index of CM patients was significantly higher than healthy controls (1.766 ± 0.209 vs. 1.633 ± 0.138, p=0.023 (Figure 2A), but there was no significant difference in the left ALPS index (p=0.711). And the right ALPS index of CM patients was significantly higher than the left ALPS index (1.766 ± 0.209 vs. 1.636 ± 0.155, p = 0.005) (Figure 2B). There was no significant correlation between the ALPS index and the clinical characteristics of CM (Table 2).Discussion
Our study first evaluated the ALPS index of CM patients and demonstrated the abnormal glymphatic function in CM patients. But as opposed to the lower ALPS index in neurodegenerative diseases, the significantly higher ALPS index may indicate an abnormal but not impaired glymphatic system. The vasomotion dysfunction associated with the pathophysiology of migraine affects the convective flow and diffusion along perivascular space. CM induces the persistent release of neuropeptides such as calcitonin gene-related peptide (CGRP), a potent vasodilator, which may, like alcohol, another vasodilator, enhance the diffusion of the metabolic waste products towards perivascular space by increasing the interactive reactivity of vascular endothelial-smooth muscle cells11,12, but compensating improvements in water diffusivity may not be sufficient to eliminate all headache-related factors, thus leading to the occurrence of a headache. Additionally, consistent with the lateralized presentations of headache in terms of functional connectivity, metabolism, and cerebral perfusion13-16, the right-prominent alterations in glymphatic function support that right dominance might be a certain feature of migraine, but longitudinal studies are needed to confirm this assumption.Conclusion
The significantly increased right ALPS index suggests the abnormal glymphatic function in CM and the right-prominent abnormality may be a feature of CM.Acknowledgements
We are most grateful for the support from the Tiantan Neuroimaging Center of Excellence, China National Clinical Research Center for Neurological Diseases, and the support of physicians at the Headache Center, Department of Neurology, Beijing Tiantan Hospital.References
1. Natoli JL, Manack A, Dean B, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010;30(5):599-609.
2. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Science translational medicine. 2012;4(147):147ra111.
3. Schain AJ, Melo-Carrillo A, Strassman AM, Burstein R. Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache. J Neurosci. 2017;37(11):2904-2915.
4. Yi T, Gao P, Zhu T, Yin H, Jin S. Glymphatic System Dysfunction: A Novel Mediator of Sleep Disorders and Headaches. Front Neurol. 2022;13(885020.
5. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science (New York, NY). 2013;342(6156):373-377.
6. Lee DA, Lee HJ, Park KM. Normal glymphatic system function in patients with migraine: A pilot study. Headache. 2022;62(6):718-725.
7. Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol. 2017;35(4):172-178.
8. Zhang W, Zhou Y, Wang J, et al. Glymphatic clearance function in patients with cerebral small vessel disease. Neuroimage. 2021;238(118257.
9. Irfanoglu MO, Nayak A, Jenkins J, Pierpaoli C. ,TORTOISEv3:Improvements and New Features of the NIH Diffusion MRI Processing Pipeline, 2017, ISMRM 25th annual meeting, Honolulu, HI, abstract #3540
10. Pierpaoli C, Walker L, Irfanoglu MO, et al. TORTOISE: an integrated software package for processing of diffusion MRI data. 2010, ISMRM 18th annual meeting, Stockholm, Sweden, abstract #1597
11. Cheng Y, Liu X, Ma X, Garcia R, Belfield K, Haorah J. Alcohol promotes waste clearance in the CNS via brain vascular reactivity. Free radical biology & medicine. 2019;143(115-126.
12. Iyengar S, Ossipov MH, Johnson KW. The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158(4):543-559.
13. Zhang D, Huang X, Mao C, et al. Assessment of normalized cerebral blood flow and its connectivity with migraines without aura during interictal periods by arterial spin labeling. J Headache Pain. 2021;22(1):72.
14. Michels L, Villanueva J, O'Gorman R, et al. Interictal Hyperperfusion in the Higher Visual Cortex in Patients With Episodic Migraine. Headache. 2019;59(10):1808-1820.
15. Bathel A, Schweizer L, Stude P, et al. Increased thalamic glutamate/glutamine levels in migraineurs. J Headache Pain. 2018;19(1):55.
16. Amin FM, Hougaard A, Magon S, et al. Altered thalamic connectivity during spontaneous attacks of migraine without aura: A resting-state fMRI study. Cephalalgia. 2018;38(7):1237-1244.
Figures