3184
Characteristics of cerebral blood flow in sleep deprivation based on arterial spin labeling
Chen Wang1, Xiaolei Wang1, Leilei Li1, and Yuanqiang Zhu1
1Department of Radiology, Xijing Hospital, Air Force Medical University, Xi'an, China
1Department of Radiology, Xijing Hospital, Air Force Medical University, Xi'an, China
Synopsis
Keywords: Neurofluids, Nervous system
ASL has been widely used in neuroimaging studies to measure the metabolic activity of local brain microstructure due to its advantages of no radiation damage, no introduction of exogenous contrast agent, high repeatability and fast scanning time.In this study, ASL perfusion imaging was used to analyze the changes of cerebral blood perfusion during normal sleep and sleep deprivation.Introduction
Acute sleep deprivation refers to sleep loss due to various reasons and changes in a series of physiological and psychological functions, thus leading to changes in various behavioral and cognitive functions of the body [1,2]. In terms of behavioral cognition, acute sleep deprivation mainly has a significant impact on working memory, design decision, motor control and attention, among which impaired sustained attention is the most common [3]. A study using positron emission tomography (PET) has found that glucose metabolism levels in the thalamus, prefrontal cortex and parietal lobe of the body are significantly decreased after sleep deprivation and are significantly correlated with cognitive performance. This suggests that impaired sustained attention after sleep deprivation may be related to decreased perfusion in relevant brain regions. PET mainly uses radioactive isotopes as tracers to obtain cerebral blood flow values. Considering the harm of isotope radioactivity to human body, the use of PET to study the mechanism of sustained attention impairment after sleep deprivation has certain limitations. As an FMRI technique that directly reflects the metabolic activity of local brain microstructure, arterial spin labeling (ASL) mainly uses arterial spin labeling of blood hydrogen proton as a self-contrast tracer to quantitatively measure microvascular perfusion and reflect the situation of cerebral blood flow (CBF) [4,5]. A number of studies have confirmed that CBF calculated by ASL has a high consistency with the blood perfusion value and glucose metabolism obtained by PET [6-8]. Another study [9] emphasized that relative cerebral blood flow (rCBF) based on ASL can display the dynamic changes of microvascular perfusion more reliably than CBF, and rCBF can eliminate the influence of individual differences in microvascular perfusion and scanning parameters to the maximum extent. Based on this, this study intends to use ASL imaging technology and rCBF obtained based on standardized processing to conduct statistical analysis of whole brain voxel, explore the similarities and differences between cerebral blood perfusion patterns under sleep deprivation and rest wakefulness, and evaluate the correlation between changes in perfusion patterns and changes in sustained attention.Methods
In this experiment, 54 college students (including undergraduates and postgraduates) were recruited by means of recruitment information released in various universities in Xi 'an, including 29 male and 25 female volunteers, with an average age of 22.46±1.81 years old.Each volunteer underwent a total of two MRI scans after normal sleep and sleep deprivation, both set at 8 a.m.The psychomotor vigilance test (PVT) was used to assess sustained attention after normal sleep or sleep deprivation.Scanning parameters: 3D pcASL: TR = 4844 ms, TE = 10.5ms, post labeling delay time (PLD) = 2025 ms, layer thickness 4.0mm, continuous scanning without interval, FOV 240mm×240mm, matrix 128×128, etc. Layer number 36, collection times 3. 3D T1WI: TR = 12 ms, TE = 5.1 ms, layer thickness 1.0mm, layer number 176, continuous scanning without interval, FOV 256 mm×256 mm, matrix 256×256.Results
3.1 Comparative analysis of cerebral blood perfusion after sleep deprivation and rest wakefulnessThe paired sample T-test was used to analyze the difference in rCBF value between sleep deprivation and normal sleep. The results showed that, compared with normal sleep, after sleep deprivation, blood perfusion in bilateral dorsolateral prefrontal cortex, bilateral apex lobules, left orbital middle frontal gyrus, bilateral middle temporal gyrus, right posterior central gyrus and bilateral angular gyrus were significantly decreased (t < -2.78, P< 0.05, FDR correction). However, blood perfusion increased significantly in bilateral thalamus, left precuneus, right medial prefrontal lobe, left posterior cingulate gyrus and left inferior temporal gyrus (t > 2.67, P< 0.05, FDR correction).
3.2 Correlation analysis of rCBF value and PVT task in abnormal brain regions of sleep deprivation
In order to investigate the relationship between the changes of blood perfusion in the relevant brain areas and the changes of sustained attention after sleep deprivation, Pearson correlation analysis was used to calculate the correlation between the variation amplitude of rCBF and the difference of PVT leakage frequency in the above different brain areas after sleep deprivation and rest wakefulness. The results showed that the rCBF of the left dorsolateral prefrontal lobe and the right apex lobule was negatively correlated with the difference of PVT leakage frequency (r=-0.56, P<0.001; r=-0.64, P<0.001), the changes of rCBF in the left precuneus were positively correlated with the difference of PVT leakage frequency (r=0.72, P<0.001), and the changes of rCBF in the other brain regions were not significantly correlated with the difference of PVT leakage frequency.
Acknowledgements
No acknowledgement found.References
[1] Liew S C, Aung T.Sleep deprivation and its association with diseases- a review[J].Sleep Med,2021, 77: 192-204. DOI:10.1016/j.sleep.2020.07.048. [2] Atrooz F, Salim S.Sleep deprivation, oxidative stress and inflammation[J].Adv Protein Chem Struct Biol,2020, 119: 309-336. DOI:10.1016/bs.apcsb.2019.03.001. [3] Hudson A N, Van Dongen H P A, Honn K A.Sleep deprivation, vigilant attention, and brain function: a review[J].Neuropsychopharmacology,2020, 45 (1): 21-30. DOI:10.1038/s41386-019-0432-6. [4] Zhao M Y, Václavů L, Petersen E T, et al.Quantification of cerebral perfusion and cerebrovascular reserve using Turbo-QUASAR arterial spin labeling MRI[J].Magn Reson Med,2020, 83 (2): 731-748. DOI:10.1002/mrm.27956. [5] Lin T, Qu J, Zuo Z, et al.Test-retest reliability and reproducibility of long-label pseudo-continuous arterial spin labeling[J].Magn Reson Imaging,2020, 73: 111-117. DOI:10.1016/j.mri.2020.07.010. [6] Scott C J, Jiao J, Melbourne A, et al.Reduced acquisition time PET pharmacokinetic modelling using simultaneous ASL-MRI: proof of concept[J].J Cereb Blood Flow Metab,2019, 39 (12): 2419-2432. DOI:10.1177/0271678x18797343. [7] Rischka L, Godbersen G M, Pichler V, et al.Reliability of task-specific neuronal activation assessed with functional PET, ASL and BOLD imaging[J].J Cereb Blood Flow Metab,2021, 41 (11): 2986-2999. DOI:10.1177/0271678x211020589. [8] Wang J, Sun H, Cui B, et al.The Relationship Among Glucose Metabolism, Cerebral Blood Flow, and Functional Activity: a Hybrid PET/fMRI Study[J].Mol Neurobiol,2021, 58 (6): 2862-2873. DOI:10.1007/s12035-021-02305-0.Figures
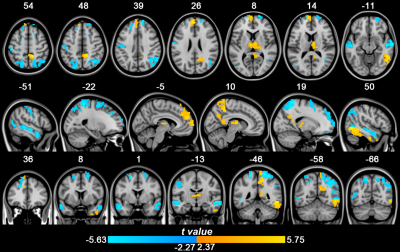
Figure 1. Paired T-test results of rCBF values in the whole brain after sleep deprivation and rest
wakefulness
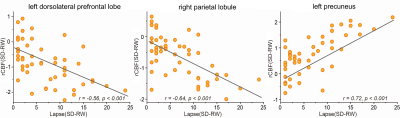
Figure 2. Correlation analysis of rCBF value and PVT task in the Left dorsolateral prefrontal lobe, right parietal lobule and left precuneus
RW: rest wakefulness, SD: sleep deprivation
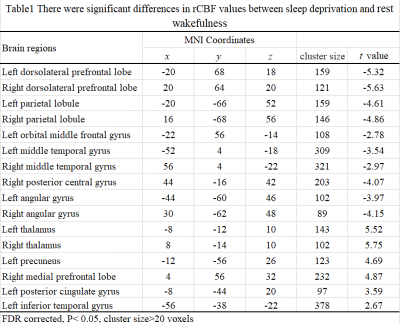
Table1. There were significant differences in rCBF values between sleep deprivation and
rest wakefulness
DOI: https://doi.org/10.58530/2023/3184