3180
The potential of ultra-high resolution 7T T2-weighted TSE to assess the glymphatic system’s structure1Biomedical Magnetic Resonance, Otto von Guericke University, Magdeburg, Germany, 2German Center for Neurodegenerative Disease, Magdeburg, Germany, 3Center for Behavioral Brain Sciences, Magdeburg, Germany, 4Leibniz Institute for Neurobiology, Magdeburg, Germany
Synopsis
Keywords: Data Acquisition, Neurofluids
In this proof-of-principle study the currently untapped potential of UHF MRI is shown. Two young, healthy volunteers were scanned at 7T with 2D 0.25x0.25x1.0mm T2w TSE depicting a substantial number of PVS. At this high resolution, vessel segments inside the PVS could be studied and quantified. Further, hyperintense structures around and inside the dura and sinuses were depicted clearly.
Introduction
The majority of studies assessing the glymphatic system are performed at clinically available field strengths to obtain larger cohort sizes1,2. Although essential for the advancement of the field, the potential of ultra-high field (UHF) MRI to image the glymphatic system at ultra-high resolution has not been fully assessed. To that end, a proof-of-principle study to fully non-invasively assess perivascular spaces (PVS) and dura around the sinus was performed using T2-weighted Turbo-Spin-Echo (TSE) sequence at 7T.Methods
Two male volunteers were scanned at 7T using a 32-channel head coil. For both volunteers, 2D, non-contrast enhanced T2-weighted TSE protocols with 0.25x0.25x1.0mm voxel size (i.e. 0.0625µl voxel volume) were acquired. While for the 38 years old volunteer a single average of a 30 mm thick axial slab was acquired (7:07 min), for the 27 years old volunteer, two averages of an 18 mm thick coronal slab were acquired (15:32 min). Further, for the 27 years old volunteer prospective motion correction using a marker attached to a mouth piece and an in-bore optical tracking system3 was applied to minimize motion-induced image blurring. For the 38 years old volunteer, vessels insight PVS could be depicted. These vessels and their respective PVS were manually segmented and the vessel-to-PVS ratio as well as the ratio of vessel volume to PVS volume were computed.Results
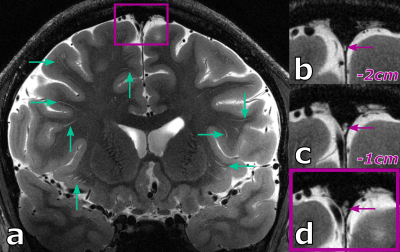
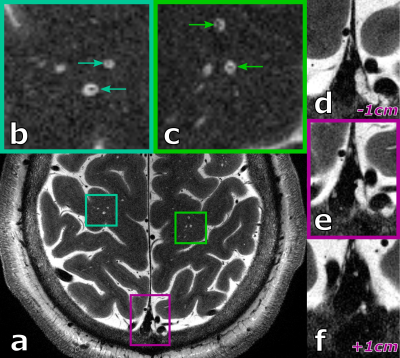
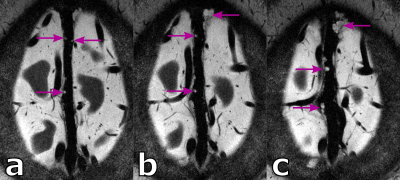
When using the ultra-high resolution capabilities of UHF MRI, even in healthy, young volunteers a substantial amount of PVS can be depicted (see 27 years old volunteer in Fig.1). Further, PVS propagating from deep white matter to the gray matter boundary can be observed clearly. Besides PVS, hyperintense structures around the parasagittal dura can be seen. These structures represent potentially lymphatic vessels, granulations, or vessel wall segments inside the sinus.For the 38 years old volunteer (see Fig.2-4) similar hyperintense structures in the sinus and a noteworthy amount of PVS can be seen. With the ultra-high resolution used in this study, in five PVS the corresponding vessel inside the PVS itself can be depicted at least partially. This is an observation uncommon at lower resolutions and, to our best knowledge not described in the literature to date. For the five spaces with a vessel inside, the average ± standard deviation was 1.62 ± 0.14 and 0.11 ± 0.05 for the vessel-to-PVS signal ratio and vessel-to-PVS volume ratio, respectively. Hence, only a minor volume fraction of the PVS with vessels is actually occupied by the vessel. While the signal ratio for the detected vessel segment is sufficient for detection, the remaining vessel segments are no depicted, most likely due to partial volume effects. This might be in part driven by the anisotropic resolution. In the thick slices, the majority of detected vessel segments was oriented perpendicular to the slices. Hence, for sufficient vessel-to-PVS ratio independent of the vessel and slice orientation isotropic resolutions are favorable.
Discussion
This proof-of-principle study showed the currently untapped potential of UHF MRI in two young, healthy volunteers. Although only healthy volunteers were scanned, a substantial number of PVS was detected, indicating the need of interpreting PVS counts and scores with respect to the imaging resolution used when comparing results across studies. Further, vessel segments inside the PVS could be studied and quantified. By using isotropic resolutions, ideally with 3D SPACE sequences instead of 2D TSE, more robust depiction of PVS and related vessels could be established. With such imaging capabilities and the on-going translation of UHF MRI to the clinic, PVS could be quantified by the ratio of vessel-to-PVS volume, hence, quantifying how enlarged the space actually is. Beyond PVS; granulations and other hyperintense features around the parasagittal sinus could be depicted. Comparison with ultra-high resolution, contrast-enhanced protocols are required to fully understand what these hyperintense structures around and inside the sinus represent and what their function in glymphatic system is. Potentially, UHF MRI at ultra-high resolutions could represent an alternative to study the structure of the glympathic system without the need for contrast agent. Hence, 7T, T2-weighted TSE could be a fully non-invasive alternative to contrast-enhanced protocols and would render the need for contrast agents and long delays between contrast admission and image acquisition obsolete when 7T is available.Acknowledgements
This work was funded by the DFG-MA 9235/1-1 and MA 9235/3-1References
1. Ringstad, Geir, et al. "Brain-wide glymphatic enhancement and clearance in humans assessed with MRI." JCI insight 3.13 (2018).
2. Taoka, Toshiaki, and Shinji Naganawa. "Glymphatic imaging using MRI." Journal of Magnetic Resonance Imaging 51.1 (2020): 11-24.
3. Stucht, Daniel, et al. "Highest resolution in vivo human brain MRI using prospective motion correction." PloS one 10.7 (2015): e0133921.
Figures