3175
Novel contrast agents for MR based on chemical exchange saturation transfer and its potential applications1Department of Radiology, Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, Xiangyang, China, 2Department of Radiology, Second Affiliated Hospital, Shantou University Medical College, Shantou, China, 3Department of Radiology, Huizhou central people's hospital, Huizhou, China
Synopsis
Keywords: CEST & MT, Molecular Imaging
This study aimed to investigate some small molecular compounds with an appropriate chemical exchange site that can be employed as negative contrast agents for magnetic resonance imaging. 17 kinds of small molecular substances were selected and the imaging were performed on 7.0T MR system. Except for biotin, a series of small molecular substances have the characteristics of CEST. Salicylic acid (9.3ppm), 5-aminosalicylic acid (8.0ppm), and olsalazinesodium (9.8ppm) have large frequency offset, with optimal pH value 6.4~6.8, which are ideal candidates for CEST contrast agents. Due to the differences of molecular structure, the CEST characteristics were different.Introduction
Magnetic resonance imaging, a versatile and powerful imaging technique, is widely used for disease diagnosis due to its non-invasive nature, high spatial resolution, high soft tissue resolution and high penetration. However, MRI has low sensitivity and can only provide macroscopic morphological features such as the size and structure of tissues and organs. In recent years, the chemical exchange saturation transfer (CEST) molecular imaging technique, which is based on MRI, not only has the characteristics of MRI with high soft tissue resolution, but also is comparable to PET technology in molecular imaging [1, 2]. In a word, CEST technology can be used to visualize and quantify the functional and metabolic physiological and biochemical information of lesions at the molecular level. Currently, the commonly used MR contrast agents in clinical practice are mainly metal chelates containing Gd. However, these contrast agents have potential metal toxicity and T2* effects that limit their wide application[3]. CEST contrast agents are a novel class of negative contrast agents that mainly use CEST techniques to image substances with exchangeable protons, which can be labeled without metallic substances or radioactive substances, and without the risk of T2* effects and metal deposition under high dose conditions [4].Therefore, the aim of this study was to find small molecules with suitable chemical exchange sites and potential to be prepared into polymeric nanoparticles that can be used as chemical exchange saturation transfer contrast agents under physiological conditions, and to explore their potential applications.Methods
17 kinds of small molecular substances were selected and dissolved in phosphate buffered saline (PBS) to prepare phantoms. All images were performed on an Agilent 7.0 T MR system with a 9563 body coil for signal transmission and reception. An improved version of continuous wave echo planar imaging sequence was used for the CEST imaging with the following parameters: TR = 6000 ms, TE = 29.46 ms, Kzero = 32, slice thickness = 2 mm, FOV = 30 × 30 mm, matrix size = 64 × 64. All of the CEST image processing and data analyses were performed using custom written scripts in MATLAB. Statistical evaluations were performed using GraphPad Prism software.Results
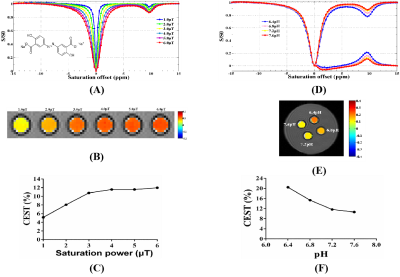
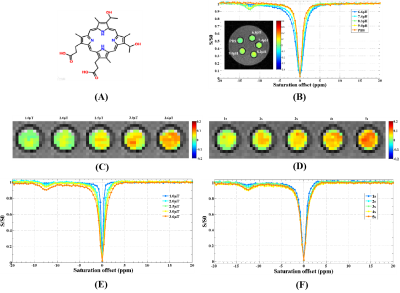
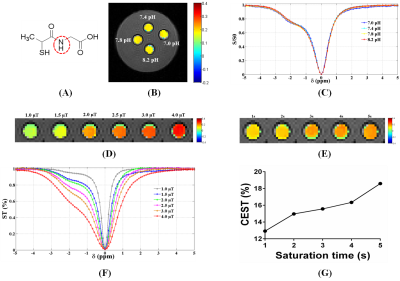
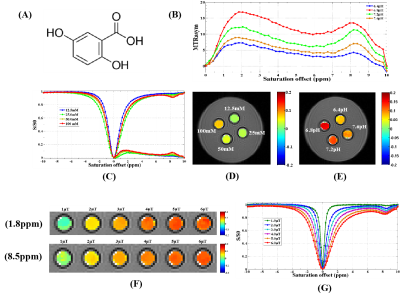
Except for biotin, a series of small molecular substances had the characteristics of CEST. The chemical shift of acrylamide is 2.75 ppm and the optimal pH, saturation power and saturation time were 7.8, 3.0 μT and 4 s, respectively. The optimal chemical exchange sites of L-phenylalanine, dopamine hydrochloride, and olsalazine sodium were at 2.65 ppm, 2.6 ppm, and 9.8 ppm, respectively. The CEST effects of its were positively correlated with the saturated power and negatively correlated with the pH value. The optimal chemical exchange sites for 4-amino-3-nitrophenol and N-(2-Mercaptopropionyl) glycine were at 1.65 ppm and -2.5 ppm, respectively, and the pH value had no significant effect on the CEST effect. 2,5-dihydroxybenzoic acid and 5-aminosalicylic acid both had two chemical exchange sites, which were at 1.8 ppm, 8.5 ppm and 1.0 ppm, 8.0 ppm, respectively. Other small molecular substances, such as salicylic acid (9.3 ppm), L-cysteine (-2.75 ppm), α-Cyclodextrin (0.5 ppm), meso-2,3-Dimercaptosuccinic acid (-2.5 ppm), Hyaluronic acid (1.0 ppm), 3,5-Dihydroxybenzamide (1.8 ppm), Folic acid (1.8 ppm), Hematoporphyrin (-12.5 ppm) and Glutathione (-2.75 ppm and 3.0 ppm), due to the differences of molecular structure, the CEST characteristics were different.Discussion
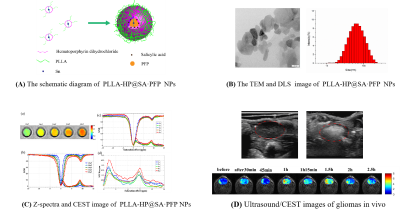
From a small group of preliminarily screened candidates, we demonstrated a class of small molecules with CEST effect can be used as a nonmetallic MR contrast agent. Based on the different CEST properties of the above small molecules, experimental designs can be targeted to fully exploit their potential applications. For example, we have successfully prepared a nanopharmaceutical imaging probe (PEG-PAM-PAN@DOX) with CEST imaging and therapeutic efficacy based on acrylamide imaging for breast cancer diagnosis and treatment studies [5]. Now, we also have successfully synthesized a bimodal nanoimaging probe (PLLA-HP@SA·PFP) with ultrasonography and CEST properties for early diagnostic studies of glioma based on salicylic acid and perfluoropentane using nanotechnology. In addition, the ability to monitor dopamine changes in the brain using CEST technology could be of great value for the study of brain cognitive function and early diagnosis of diseases. Hematoporphyrin (-12.5 ppm), Salicylic acid (9.3ppm) and analogues [5-aminosalicylic acid (8.0ppm); 5-dihydroxybenzoic acid (8.5 ppm); olsalazinesodium(9.8ppm)] have large frequency offset, which are ideal candidates for CEST contrast agents.Conclusion
These small molecules have great potential for applications in early diagnosis of diseases, targeted imaging and targeted delivery of drugs, which not only help to develop new contrast agents for use as non-invasive biomarker imaging, but also help to guide the development of novel nanomedicines.Acknowledgements
This work was supported by grants from the Key Science and Technology Projects in Health Care in Xiangyang City (2022YL15A)References
[1] VAN ZIJL P C, YADAV N N. Chemical exchange saturation transfer (CEST): what is in a name and what isn't? . Magn Reson Med, 2011, 65(4): 927-48.
[2] PARROTT D, FERNANDO W S, MARTINS A F. Smart MRI Agents for Detecting Extracellular Events In Vivo: Progress and Challenges. Inorganics, 2019, 7(2).
[3] SOESBE T C, WU Y, DEAN SHERRY A. Advantages of paramagnetic chemical exchange saturation transfer (CEST) complexes having slow to intermediate water exchange properties as responsive MRI agents. NMR Biomed, 2013, 26(7): 829-38.
[4] YUAN Y, ZHANG J, QI X, et al. Furin-mediated intracellular self-assembly of olsalazine nanoparticles for enhanced magnetic resonance imaging and tumour therapy. Nat Mater,2019, 18(12): 1376-83.
[5] JIA Y, WANG C, ZHENG J, et al. Novel nanomedicine with a chemical-exchange saturation transfer effect for breast cancer treatment in vivo. J Nanobiotechnology, 2019, 17(1): 123.
Figures