3174
Characterization and Optimization of New CEST Reporter Proteins with Increased Detection Specificity1Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Chemical Engineering and Materials Science, Michigan State University, East Lansing, MI, United States, 3Department of Pathology, Massachusetts General Hospital, Charlestown, MA, United States
Synopsis
Keywords: CEST & MT, Cell Tracking & Reporter Genes
CEST reporter genes could play a critical role in the optimization of novel biological therapeutics. However, existing amide proton based CEST reporter genes have poor specificity as the amide protons of the reporter gene have the same chemical shift as amide protons from endogenous proteins. Here we report on a new class of reporter proteins with CEST contrast at 5-ppm, based on tyrosine hydroxyl ring protons.Introduction
Cell and viral based therapeutics hold great promise for revolutionizing the treatment of many diseases. However, the optimization of such biological therapies and assessment of their efficacy depends critically on the ability to monitor the spread and persistence of the therapeutic agent. We have previously demonstrated that a Lysine-Rich Protein (LRP) Chemical Exchange Saturation Transfer (CEST) MRI-based reporter gene1 can be used for imaging oncolytic virotherapy2 as well as cardiac gene transfer therapy3. However, the detection sensitivity and specificity were limited. In particular, the amide exchangeable protons of the LRP have the same chemical shift (3.5 ppm) as amide exchangeable protons from endogenous proteins, severely limiting the specificity. Here we demonstrate a new class of CEST reporter proteins, based on bovine pancreatic trypsin inhibitor (BPTI) protein, with contrast at 5-ppm chemical shift arising from very fast exchanging ring hydroxyl protons of tyrosine residues. Mutant BPTI variants have been engineered to reduce the hydroxyl exchange rates thereby increasing the saturation efficiency and optimizing the CEST detection sensitivity.Methods
BPTI (Sigma-Aldrich) was dissolved in 1x PBS and titrated with 0.6 M HCl to a pH of 7.4, 7.0 or 6.6. Ultra-fast Z-spectroscopy4 experiments were performed on a Bruker 14T NMR spectrometer (Bruker Biospin). The pH and temperature dependence of the CEST contrast and proton exchange rates for the new 5-ppm reporter proteins were characterized by the Quantification of Exchange via Saturation Power (QUESP)5 method. Z-spectra were acquired at 10°, 20°, 30° and 37°C. The PROSS6 protein optimization tool was used to design more stable mutant proteins (mutations L29R and K41N) which should better protect the tyrosine residues and slow down the hydroxyl proton exchange rates. Genes encoding either wild-type (wt-BPTI) or mutant BPTI (m-BPTI), with an additional His tag for immunohistochemistry detection, were then engineered into BL21 bacterial cells. Dot blots and western blots of cell lysates were performed to confirm protein expression.Results and Discussion
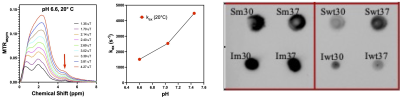
BPTI is a small protein of 58 amino acids (MW 6.5 kDa) that contains four tyrosine residues: Y10, Y21, Y23, Y35. The CEST magnetization transfer ratio asymmetry (MTRasym) of BPTI acquired at pH 6.6 and 20°C (Fig. 1, left) shows a resolved peak at 4.8-ppm assigned to the tyrosine ring hydroxyl protons. Figure 1 (middle) shows the pH dependence of the chemical exchange rate at 20°C. At neutral pH the tyrosine hydroxyl ring protons are in the fast chemical exchange regime with exchange rate constants > 2500 s-1, making them difficult to saturate efficiently and detect. At higher temperature and pH, the distinct peak at 4.8-ppm is lost due to the increased exchange rate. Stabilization of the protein structure should help to better sequester the tyrosine residues, decrease the hydroxyl proton exchange rates, and increase the CEST contrast. More stable mutant BPTI structures were designed using the PROSS6 tool and the associated reporter genes were engineered into BL21 bacterial cells. Dot blots of BL21 cell lysates confirm expression of wt-BPTI and m-BPTI in both soluble and insoluble cell fractions (Fig. 1, right). m-BPTI protein is currently being purified for CEST characterization. The fast hydroxyl proton exchange rate, even at low pH, of such a 5-ppm reporter protein would not only provide dramatically increased specificity but would also provide high detection sensitivity at the low pH observed in many pathologies.Acknowledgements
We gratefully acknowledge financial support from the National Institutes of Health grants R01-CA203873, R01-EB031008, and P41-RR14075.References
1. Gilad, A. A. et al. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol 25, 217–219 (2007).
2. Farrar, C. T. et al. Establishing the Lysine-rich Protein CEST Reporter Gene as a CEST MR Imaging Detector for Oncolytic Virotherapy. Radiology 275, 746–754 (2015).
3. Meier, S. et al. Non-invasive detection of adeno-associated viral gene transfer using a genetically encoded CEST-MRI reporter gene in the murine heart. Sci Rep 8, 4638 (2018).
4. Xu, X., Lee, J.-S. & Jerschow, A. Ultrafast scanning of exchangeable sites by NMR spectroscopy. Angew Chem Int Ed 52, 8281–8284 (2013).
5. McMahon, M. T. et al. Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): pH calibration for poly-L-lysine and a starburst dendrimer. Magn Reson Med 55, 836–847 (2006).
6. Weinstein, J. J., Goldenzweig, A., Hoch, S. & Fleishman, S. J. PROSS 2: a new server for the design of stable and highly expressed protein variants. Bioinformatics 37, 123–125 (2020).
Figures