3163
Reproducibility of pH-Weighted Chemical Exchange Saturation Transfer (CEST) Contrast in the Healthy Cervical Spinal Cord1Medical Biophysics, Western University, London, ON, Canada, 2Centre for Functional and Metabolic Mapping, Robarts Research Institute, London, ON, Canada, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Clinical Neurological Sciences, University Hospital, London Health Sciences Centre, London, ON, Canada
Synopsis
Keywords: CEST & MT, Spinal Cord
Ischemia and hypoxia can occur in the spinal cord due to several conditions, including compression and injury; however, in-vivo measurements of ischemia have been challenging. Chemical Exchange Saturation Transfer (CEST) can produce pH-weighted contrast, which is an indicator of tissue hypoxia. The purpose of this study was to optimize pH-weighted CEST contrast in the healthy cervical spinal cord using a prototype 3D CEST sequence on a 3T Siemens Prisma Fit MRI and determine the reproducibility of measurements at various levels along the cervical spinal cord.Introduction
Ischemia and hypoxia in the spinal cord can be caused by several factors, including compression, injury, vascular alterations, and muscular sclerosis. Unfortunately, it is challenging to measure spinal cord ischemia and hypoxia non-invasively using MRI methods due to the small size of the spinal cord, surrounding bony structure, and respiratory/cardiac motion. Chemical Exchange Saturation Transfer (CEST) is an MRI contrast that exploits the transfer of magnetic saturation from selectively excited endogenous protons to bulk water protons,1 resulting in a reduction in the observed water signal.2 pH-weighted CEST contrasts are produced by exploiting the pH dependence of the proton exchange rate. Since hypoxia alters tissue pH, pH-weighted CEST images could provide an indirect measurement of tissue ischemia and hypoxia. Previously, it has been shown that pH-weighted CEST contrast can be generated in the brain using a ratiometric method called amine/amide concentration-independent detection (AACID).3 The objective of this study was to optimize sequence parameters to maximize CEST contrast at 3T in the spinal cord and then evaluate the reproducibility of the AACID measurement along different levels of the healthy cervical spinal cord using a 3D CEST sequence.Methods
On a 3T Siemens MAGENTOM Prisma Fit MRI scanner, a prototype CEST sequence was used that consisted of a pre-saturation scheme followed by a 3D gradient-echo readout using centric spiral reordering. The number, type, and duration of saturation pulses were varied to maximize the CEST contrast in the human brain in three healthy participants. This optimal saturation scheme was applied in the spinal cord at 62 offsets from -6.5 to 6.5 ppm. Other relevant imaging parameters included: axial orientation, TR/TE = 3.35/1.16 ms, matrix size = 96 x 96, 14 slices, resolution = 2.0 x 2.0 x 5.0 mm3, GRAPPA acceleration factor = 2. To correct for B0 inhomogeneities on a pixel-by-pixel basis, the CEST spectrum was shifted back to 0 ppm using a Lorentzian fitted water saturation shift referencing (WASSR) spectrum,4 acquired with a pulse train of five Gaussian-shaped pulses (same sequence parameters as above, 25 frequency offsets from -2.0 to 2.0 ppm). In the spinal cord, 16 non-saturated scans were interleaved throughout the acquisition,5 the respiratory cycle was collected using respiratory bellows and then used together to correct for the global effect of respiration, previously implemented by By et al.6 Using MATLAB, CEST spectra were fitted pixel-by-pixel with a six-pool Lorentzian model (water, amide, amine (2.0 ppm), amine (2.75 ppm), nuclear Overhauser enhancement, magnetization transfer). To determine the reproducibility of CEST contrast in the spinal cord, 12 healthy participants (7 females, mean age (±SD) 26 ± 4 years) were scanned at two different time points (mean repeat time (±SD) 10 ± 4 days) with the CEST acquisition centered at level C4 in the cord. T2-weighted anatomical cervical spine images were acquired and semi-automatically segmented and labelled (C3-C5) using the Spinal Cord Toolbox (SCT).7 The average AACID values for each level of the cervical spinal cord were calculated and compared (Repeated One-Way ANOVA, corrected for multiple comparisons (Tukey), p<0.05). To quantify the reliability of the AACID measurement at levels C3, C4, and C5, the intraclass correlation (ICC) coefficient was calculated.Results
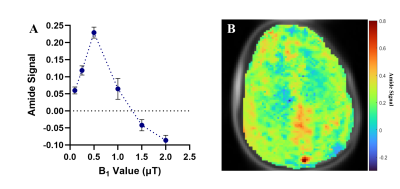
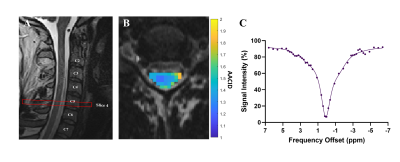
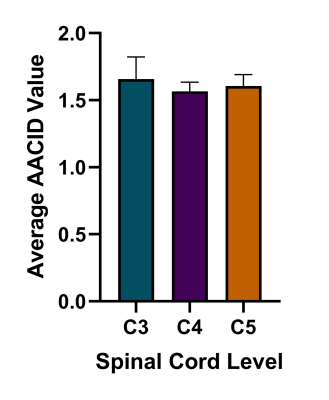
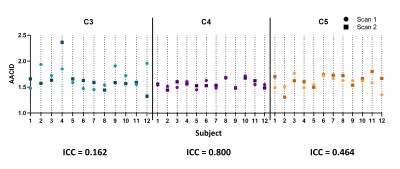
The optimal saturation scheme consisted of 30 Gaussian-shaped radiofrequency (RF) pulses with pulse length = 100 ms, and interpulse delay = 1 ms. The optimal amide CEST effect was achieved at a B1 value of 0.5 µT (Figure 1). CEST spectra in the cord clearly showed the amide proton CEST effect and were used to successfully produce AACID maps in the cord (Figure 2). There was no significant difference in the average AACID values between spinal cord levels C3, C4, and C5 (Figure 3). The reliability between the calculated AACID values of scan 1 and scan 2 was characterized by an ICC value of 0.162 for spinal cord level C3, 0.800 for level C4, and finally, 0.464 for spinal cord level C5 (Figure 4).Discussion
The optimized CEST sequence produced high-quality images in the cervical spinal cord in healthy participants. No difference in the calculated AACID values was found between any spinal cord levels, suggesting that AACID values do not significantly change throughout the healthy cervical spinal cord. The reproducibility of the AACID measurements between scans separated by 10 days in healthy participants was poor at C3, good at C4, and fair at C5. The reproducibility of AACID values was highest at the C4 level, suggesting that the center slices of the 3D CEST sequence produce the most consistent AACID measurements.Conclusion
CEST contrast in the spinal cord has been previously demonstrated.6,8 However, the current study utilized a prototype 3D CEST sequence at 3T to evaluate AACID reproducibility in healthy cervical spinal cords. The spinal cord level C4 had the best reproducibility, corresponding to the center of the 3D acquisition. This result suggests that applications of this 3D CEST sequence should be centered on the lesion or region of interest to achieve the most accurate AACID measurements. In the future, patients with spinal cord injury or compression will be studied to determine if pH-weighted AACID measurements can detect pH heterogeneity in the spinal cord in these conditions.Acknowledgements
We thank Scott Charlton and Oksana Opalevych (CFMM, Robarts Research Institute, The University of Western Ontario) for facilitating MRI acquisitions.
References
[1] Sherry A, Woods M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu Rev Biomed Eng. 2008; 10(3/4): 391-411.
[2] van Zijl PCM, Yadav NN. Chemical Exchange Saturation Transfer (CEST): what is in a name and what isn’t? Magn Reson Med. 2011; 65(4): 927-948.
[3] McVicar N, Li AX, Goncalves DF, Bellyou M, Meak SO, Prado MAM, Bartha R. Quantitative tissue pH measurement during cerebral ischemia using amine and amide concentration-independent detection (AACID) with MRI. J Cereb Blood Flow Metab. 2014; 34: 690-698.
[4] Kim M, Gillen J, Landman BA, Zhou J, van Zijl PCM. Water Saturation Shift Referencing (WASSR) for Chemical Exchange Saturation Transfer (CEST) Experiments. Magn Reson Med. 2009; 61: 1441-1450.
[5] Jones CK, Huang A, Xu J, et al. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. NeuroImage. 2013; 77: 114-124.
[6] By S, Barry RL, Smith AK, et al. Amide Proton Transfer CEST of the Cervical Spinal Cord in Multiple Sclerosis Patients at 3T. Magn Reson Med. 2018; 79: 806-814.
[7] De Leener B, Lévy S, Dupont SM, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 2017; 145: 24–43.
[8] Dula AN, Pawate S, Dethrage LM, Conrad BN, Dewey BE, Barry RL, Smith SA. Chemical exchange saturation transfer of the cervical spinal cord at 7T. NMR Biomed. 2016; 29: 1249-1257.
Figures