3150
Building a Liver Perfusion Phantom for Vessel Size Imaging
Dominick Jon Romano1, Mert Şişman2,3, Qihao Zhang1,3, Thanh Nguyen3, Pascal Spincemaille3, Martin Prince3,4, and Yi Wang1,3
1Biomedical Engineering, Cornell University, Ithaca, NY, United States, 2Electrical & Computer Engineering, Cornell University, Ithaca, NY, United States, 3Radiology, Weill Cornell Medical College, New York, NY, United States, 4Radiology, Columbia University Vagelos College of Physicians and Surgeons, New York, NY, United States
1Biomedical Engineering, Cornell University, Ithaca, NY, United States, 2Electrical & Computer Engineering, Cornell University, Ithaca, NY, United States, 3Radiology, Weill Cornell Medical College, New York, NY, United States, 4Radiology, Columbia University Vagelos College of Physicians and Surgeons, New York, NY, United States
Synopsis
Keywords: Liver, Vessels
We have developed a methodology of preparing explant livers for perfusion and vessel size imaging. Off-label use of ferumoxytol is known to provide high quality vessel size maps in vivo; however, known adverse reactions to ferumoxytol may hinder adoption into the clinical workflow. In this case, gadolinium may be more attractive. VSI experiments on a gadolinium perfused liver provide a proof of concept that Gadolinium may be used as an alternative contrast for vessel size imaging.Introduction
Vessel Size Imaging (VSI) is a technique that leverages the $$$T_{2}$$$ and $$$T_{2}^{*}$$$ relaxation properties of intravascular contrast agents to estimate vascular caliber1,2. This technique has shown good correlation with numerical simulations and histopathological measurements3-6. Furthermore, previous works have shown promise in using VSI to evaluate angiogenic changes in tumors3-8. There is further promise of using VSI in the liver to evaluate tumor progression8 and to potentially inform Interventional Radiologists on the tumor lung shunt likelihood when planning a trans arterial radioembolization of the tumor. To this end, we found it necessary to devise a workflow to prepare liver explants for vessel size imaging phantom experiments.Theory
The vessel size $$$R_{v}$$$ is determined form the following theoretical result1,2: $$R_{v}=1.602(\frac{D}{\gamma \Delta \chi B_{0}})^{1/2}(\frac{\Delta R_{2}^{*}}{\Delta R_{2}})^{3/2}$$ Where $$$R_{2}=1/T_{2}$$$ and $$$R_{2}^{*}=1/T_{2}^{*}$$$ and $$$\Delta R_{2}, \Delta R_{2}^{*}$$$ are the changes in relaxation rates due to the introduction of a superparamagnetic contrast agent. Let $$$\delta \omega = \gamma \chi B_{0}$$$, where $$$\delta \omega$$$ is approximated by measuring the contrast induced susceptibility change in a large feeding vessel. The diffusion coefficient $$$D$$$ is assigned a literature measurement9 for the liver explant, while DWI was included for the human ferumoxytol (off-brand use) scan. For the given pre and post contrast relaxation maps, the vessel size was estimated by the following inverse problem $$R^{2} = \underset{x}{\operatorname{argmin}}\lVert \delta \omega (\Delta R_{2})^{3}x – 1.062^{2}D(\Delta R_{2}^{*})^{3} \rVert_{2}^{2} + \lambda \lVert \nabla x \rVert_{2}^{2}$$ $$ R_{v}=\sqrt{R^{2}}$$Methods
The liver explant should be obtained with intact vasculature. To prepare the sample, one will need a need a Leuer-lock IV extension, a 5-inch needle driver, a razor or scalpel, pointed dressing forceps, and 4.5 inch surgical scissors (Figure 1). When examining the sample, it is best to find the largest vascular input, either the hepatic artery branch or portal veinous branch (Figure 1). Once the vascular input is found, the wide-end of the Leuer-lock may be cannulated into the vessel as shown in (Figure 1). After preliminary cannulation, the line is secured with a series of sutures (Figure 2). The suture is fed such that the material will enclose the cannulation at tying.Once the cannulated line is watertight, it is mounted onto a porous platform to allow for fluid leakage. Then, the platform is mounted onto a plastic container. The explant is then covered and may be imaged. The Leuer-lock line allows for easy connections to an IV line. An infusion pump then drives the IV line flow for the duration of the experiment.
During imaging, a flow rate of 400mL/hr is selected to perfuse the explant over the entire duration of the protocol. The theory requires pre and post-contrast measurements of $$$R_{2}$$$ and $$$R_{2}^{*}$$$. As such, the imaging protocol is defined by performing $$$R_{2}$$$, $$$R_{2}^{*}$$$, and QSM before and after contrast injection. The scanning parameters for the $$$R_{2}$$$ mapping sequence: nTE=2, TE=[9.992,99.2]ms, TR=2000ms, echo train=15, Nfreq/Nphase=256/26; Phase FOV=0.75, dz=5mm, Nz=28. For the $$$R_{2}^{*} mapping: nTE=6, TE=3.156—28.196ms, TR=170ms, flip angle=15˚,Nfreq/Nphase=256/256, Phase FOV=0.75, dz=5mm, Nz=28. For QSM: nTE=6, TE1=2.3ms, echo spacing=2.43ms, TR=16.41ms, flip angle = 5˚,Nfreq/Nphase=256/256, Phase FOV=0.75, dz=5mm, Nz=28.
For a human subject scan the scanning parameters for DWI were defined as follows: TE=7634ms, TR=2000ms, flip angle=90˚, b=[0,200,800] s/mm2, Nfreq/Nphase=128/128, Freq/Phase interpolation=2, Phase FOV=0.75, dz=5mm, Nz=2.
In the ex-vivo experiment, the liver was perfused either with Gadolinium or ferumoxytol. The ferumoxytol (30mg/ml) was diluted by 1:500 during the steady state phase, where the Gadolinium contrast was diluted by 1:20 for the steady state imaging. In the human subject, ferumoxytol (off-brand use) was dosed at 3mg/kg, diluted by 1:5, and injected at a rate of 0.6mg/s and flushed with 15mL saline at 0.1mL/s. The off-brand ferumoxytol infusion was overseen by an attending interventional radiologist, nursing, and MRI tech staff.
Results
Notice that the vessel size is only sensitive to areas of the liver where enough contrast is perfused to change the relaxation in the voxel (Figure 3,4). This is reproduced well in the case of the human subject scan (Figure 5). In this particular subject, the low VSI in the posterior portion of the right lobe is low due to a previous radioembolization treatment of the tumor.Discussion
We provide a proof of concept for designing a liver perfusion phantom for vessel size imaging. Although ferumoxytol is the standard contrast agent for VSI, we also provide a preliminary result suggesting that Gadolinium may be used to compute the vessel size in humans. This has the potential to eliminate the adverse risks associated with using ferumoxytol10 which could potentially allow for easier adoption of VSI for cancer imaging. As a future step, we plan to validate our phantoms and technique with measuring vessel caliber from micro CT and histopathology.Acknowledgements
This work was supported in part by the NIH R01NS105144, and NMSS RR-1602-07671.References
- 1. Kiselev VG, Strecker R, Ziyeh S, Speck O, Hennig J. Vessel size imaging in humans. Magn Reson Med 2005;53(3):553-563.
- Troprès I, Grimault S, Vaeth A, Grillon E, Julien C, Payen JF, Lamalle L, Décorps M. Vessel size imaging. Magn Reson Med 2001;45(3):397-408.
- Chakhoyan A, Yao J, Leu K, Pope WB, Salamon N, Yong W, Lai A, Nghiemphu PL, Everson RG, Prins RM, Liau LM, Nathanson DA, Cloughesy TF, Ellingson BM. Validation of vessel size imaging (VSI) in high-grade human gliomas using magnetic resonance imaging, image-guided biopsies, and quantitative immunohistochemistry. Scientific Reports 2019;9(1):2846.
- Farrar CT, Kamoun WS, Ley CD, Kim YR, Kwon SJ, Dai G, Rosen BR, di Tomaso E, Jain RK, Sorensen AG. In vivo validation of MRI vessel caliber index measurement methods with intravital optical microscopy in a U87 mouse brain tumor model. Neuro-Oncology 2010;12(4):341-350.
- Kellner E, Breyer T, Gall P, Müller K, Trippel M, Staszewski O, Stein F, Saborowski O, Dyakova O, Urbach H, Kiselev VG, Mader I. MR evaluation of vessel size imaging of human gliomas: Validation by histopathology. J Magn Reson Imaging 2015;42(4):1117-1125.
- Persigehl T, Ring J, Budny T, Hahnenkamp A, Stoeppeler S, Schwartz LH, Spiegel HU, Heindel W, Remmele S, Bremer C. Vessel size imaging (VSI) by robust magnetic resonance (MR) relaxometry: MR-VSI of solid tumors in correlation with immunohistology and intravital microscopy. Mol Imaging 2013;12(7):1-11.
- Foda A, Kellner E, Gunawardana A, Gao X, Janz M, Kufner A, Khalil AA, Geran R, Mekle R, Fiebach JB, Galinovic I. Differentiation of Cerebral Neoplasms with Vessel Size Imaging (VSI). Clinical Neuroradiology 2022;32(1):239-248.
- Fredrickson J, Serkova NJ, Wyatt SK, Carano RA, Pirzkall A, Rhee I, Rosen LS, Bessudo A, Weekes C, de Crespigny A. Clinical translation of ferumoxytol-based vessel size imaging (VSI): Feasibility in a phase I oncology clinical trial population. Magn Reson Med 2017;77(2):814-825.
- Do RK, Chandarana H, Felker E, Hajdu CH, Babb JS, Kim D, Taouli B. Diagnosis of liver fibrosis and cirrhosis with diffusion-weighted imaging: value of normalized apparent diffusion coefficient using the spleen as reference organ. AJR Am J Roentgenol 2010;195(3):671-676.
- Nguyen K-L, Yoshida T, Kathuria-Prakash N, Zaki IH, Varallyay CG, Semple SI, Saouaf R, Rigsby CK, Stoumpos S, Whitehead KK, Griffin LM, Saloner D, Hope MD, Prince MR, Fogel MA, Schiebler ML, Roditi GH, Radjenovic A, Newby DE, Neuwelt EA, Bashir MR, Hu P, Finn JP. Multicenter Safety and Practice for Off-Label Diagnostic Use of Ferumoxytol in MRI. Radiology 2019;293(3):554-564.
Figures
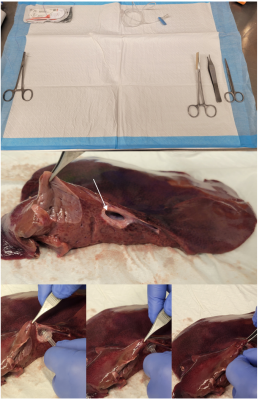
(Top) Necessary materials to cannulate a liver explant. At the left, one can find the needle driver loaded with a needle attached to 3-0 monocryl. At the center, you can find the Leuer-lock IV extension, and to the right, you can locate the razor, dressing forceps, and scissors. (Middle) Locating the largest arterial/portal vein inlet (white arrow). (Bottom) Demonstration of how to cannulate the selected vascular branch.
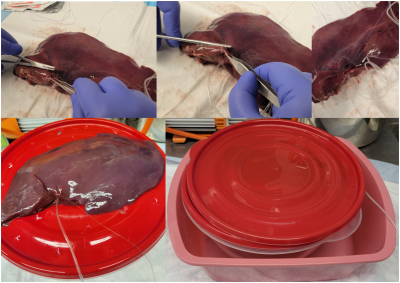
(Top) A demonstration of basic suturing. The needle is driven from a starting point through to an exit point. Then, as the needle exits, the needle point may be grabbed by the forceps to finish the pull through. This process is repeated until the cannula is surrounded by suture material, and then the suture may be tied off. (Bottom, Left) The explant is placed on top of a draining platform. (Bottom, Right) The explant is covered in solid plastic to protect the coil. Finally, a towel is draped over the setup to further absorb any fluid leakage.
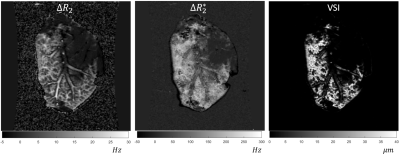
Vessel size imaging result from ferumoxytol perfused liver explant
experiment.
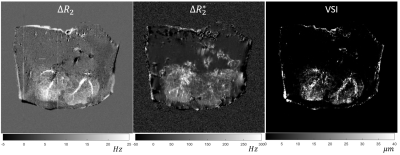
Vessel size imaging result from gadolinium perfused liver explant
experiment.
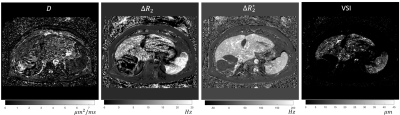
Human vessel size imaging from ferumoxytol injection. The tumor located on the anterior portion of the right lobe is dark on the VSI due to a prior radioembolization treatment.
DOI: https://doi.org/10.58530/2023/3150