3124
Subject-specific analysis reveals spatially heterogeneous white matter abnormalities in sports-related concussion1Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, United States, 2Department of Neurology, Indiana University School of Medicine, Indiana University School of Medicine, Indianapolis, IN, United States, 3Department of Biostatistics and Health Data Science, Indiana University School of Medicine, Indianapolis, India, 4Michigan Concussion Center, University of Michigan, Ann Arbor, MI, United States, 5Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 6Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, United States, 7Department of Epidemiology and Biostatistics, Indiana University School of Public Health, Bloomington, IN, United States, 8Stark Neurosciences Research Initiative, Indiana University School of Medicine, Indianapolis, IN, United States
Synopsis
Keywords: Data Analysis, Traumatic brain injury, Subject-specific analysis, Heterogeneous, Concussion
Sport-related concussion (SRC) injury inclines to cause subject-specific brain region abnormalities. This study applied subject-specific analysis that accounts for inter-subject variation to investigate heterogeneity of white matter alterations in a sample from a large national multicenter study, the Concussion Assessment, Research and Education Consortium. Our results demonstrated that subject-specific white matter abnormalities in SRC can be uncovered by calculating the extreme Z-score maps based on a template generated from normally distributed diffusion tensor imaging metric values in non-contact sports controls. Our finding indicates that the SRC-induced white matter abnormalities show heterogeneous spatial distribution across participants.Introduction
In the past years, sport-related concussion (SRC) had drawn attention from a wide perspective of clinicians, researchers, sporting organizations, and student athletes1-3. SRC may result in acute and long-term consequences on brain microstructures4-7. MRI is suitable for detecting neuropathophysiological changes after SRC and monitoring post-injury progression noninvasively. Diffusion tensor imaging (DTI), one of the MRI approaches for detecting white-matter microstructures, has demonstrated the ability to identify acute changes of the brain after SRC at the group level8. One of the DTI metrics, mean diffusivity (MD) was found to be higher in concussed athletes and associated with clinical symptoms burden and recovery at acute concussion6. Each SRC injury is a consequence of unique biomechanical forces, with a highly-individualized potential impact on white matter structure resulting in subject-specific brain region abnormalities9. Therefore, the heterogeneity in SRC white matter injury is best characterized by a subject-specific analysis that accounts for inter-subject variation10-12. In this work, we applied multiscale subject-specific analysis to investigate white-matter alterations in a sample from a large national multicenter study, the Concussion Assessment, Research and Education (CARE) Consortium. Specifically, we first applied a Z-score transformation of concussed participants’ diffusion metrics by using a template of “normal” distributions created from non-contact sport control participants. We hypothesized that white matter abnormalities in the concussed participants would be highlighted by “extreme” Z-score voxels and that their spatial distribution would be heterogeneous across the brain’s white matter.Method
Study cohortsThe participants were recruited in a multisite study of the natural history of concussion conducted through the National Collegiate Athletic Association (NCAA)–Department of Defense (DoD) CARE Consortium Advanced Research Core (ARC)13. Data were collected at the University of North Carolina (UNC), the University of California Los Angeles (UCLA), Virginia Tech (VT), and University of Wisconsin-Madison (UW). Our analysis included 252 college athletes with 266 datapoints who completed the CARE protocol by September 2020. Figure 1 displays demographics for the two groups; athletes diagnosed with concussion and sex- and age-matched non–contact-sport controls. All participants received baseline clinical assessments when recruited into the study. Concussed athletes received clinical assessments previously described13 and multimodal MRI scans1 at four time points. The current study focuses on the acute time point and 24-48 hours post-injury. Diffusion imaging protocolParticipants completed diffusion MRI on a Siemens MAGNETOM 3T Tim Trio (VT, UNC, and UCLA), 3T Prisma (UNC and UCLA), or GE 750 (UW) scanners with a 12-channel (VT), 32-channel (UNC, UCLA, UW) receiver-only head coils using a single-shot spin-echo echoplanar imaging sequence with 30 diffusion-weighting directions (b= 1,000 s/mm2) and 8 b0 (b= 0 s/mm2). One of the b0 volumes was acquired with a reversed phase-encoding direction (VT, UNC, and UCLA). Image processingThe preprocessing was described previously6. The diffusion-weighted images were first denoised with the local principal component analysis approach14. Using a pair of reversed phase-encoded b0 images as reference, the diffusion-weighted images were then corrected for motion, eddy current artifacts, and geometric distortion using FSL’s eddy_openmp15. Twenty acute datapoints with large head motion were excluded by a visual inspection quality control (QC). For athletes with multiple concussions, only the first datapoint was analyzed. The fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) maps were computed with a linear fitting algorithm (FSL dtifit). Subsequently, we transformed DTI metric images to the standard space using ANTs nonlinear registration16 and smoothed them with a 4 mm full-width at half maximum Gaussian kernel. To account for the unwanted inter-site variability, a batch-effect correction tool, ComBat, was applied on the DTI maps17. These harmonized DTI metrics for the concussed participants were first Z-score transformed using the mean and standard deviation template created from the non-contact sports control group. We then thresholded subject-specific Z-score maps (|Z| > 3.29) to highlight white matter regions (i.e., extreme voxels) that differed from the non-contact sport control reference template. To validate our hypothesis, we binarized each concussed participant’s extreme Z-score map at acute concussion and summed all the maps ,which including FA, MD, AD and RD, to generate a composite extreme map.Results
Figure 2 shows individual extreme Z-score maps for the MD metric in 64 randomly selected concussed participants. Most participants had more voxels with positive than negative extreme Z-scores, which could indicate edema and inflammation. Notably, those voxels differed across participants as illustrated by the composite extreme maps for all diffusion metrics (Figure 3). Color overlays indicate that more than one participant had a significant positive or negative extreme Z-score at that voxel. The coverage of positive extreme Z-scores was greater than that of negative extreme Z-score for MD, AD, and RD metrics. Also, positive extreme Z-scores tended to overlap more across participants (e.g., in the midbrain).Conclusion
This initial study demonstrated that subject-specific white matter abnormalities in SRC can be uncovered by calculating the extreme Z-score maps based on a template generated from normally distributed DTI metric values in non-contact sports controls. No more than 11 participants out of 97 had voxels with extreme Z-scores at the same location for any DTI metric. This finding indicates that the SRC-induced white matter abnormalities show heterogeneous spatial distribution across participants.Acknowledgements
This research was supported by the Grand Alliance Concussion Assessment, Research, and Education (CARE) Consortium, funded in part by the National Collegiate Athletic Association (NCAA) and the Department of Defense (DOD). This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Combat Casualty Care Research Program, endorsed by the Department of Defense, under Award No. W81XWH1420151. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the DOD. This research was funded by R01 NS112303.References
1. Nencka AS, Meier TB, Wang Y, Muftuler LT, Wu Y-C, Saykin AJ, et al. Stability of MRI metrics in the advanced research core of the NCAA-DoD concussion assessment, research and education (CARE) consortium. Brain imaging and behavior. 2018;12(4):1121-40.
2. DeKosky ST, Ikonomovic MD, Sam Gandy M. Traumatic brain injury--football, warfare, and long-term effects. The New England journal of medicine. 2010;363(14):1293.
3. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. The Journal of head trauma rehabilitation. 2006;21(5):375-8.
4. Wu Y-C, Harezlak J, Elsaid NM, Lin Z, Wen Q, Mustafi SM, et al. Longitudinal white-matter abnormalities in sports-related concussion: A diffusion MRI study. Neurology. 2020;95(7):e781-e92.
5. Chamard E, Lassonde M, Henry L, Tremblay J, Boulanger Y, De Beaumont L, et al. Neurometabolic and microstructural alterations following a sports-related concussion in female athletes. Brain injury. 2013;27(9):1038-46.
6. Mustafi SM, Harezlak J, Koch KM, Nencka AS, Meier TB, West JD, et al. Acute white-matter abnormalities in sports-related concussion: a diffusion tensor imaging study from the NCAA-DoD CARE Consortium. Journal of neurotrauma. 2018;35(22):2653-64.
7. Lancaster MA, Meier TB, Olson DV, McCrea MA, Nelson LD, Muftuler LT. Chronic differences in white matter integrity following sport‐related concussion as measured by diffusion MRI: 6‐Month follow‐up. Human brain mapping. 2018;39(11):4276-89.
8. Gardner A, Kay-Lambkin F, Stanwell P, Donnelly J, Williams WH, Hiles A, et al. A systematic review of diffusion tensor imaging findings in sports-related concussion. Journal of neurotrauma. 2012;29(16):2521-38.
9. Bigler ED. Neuropathology of mild traumatic brain injury: correlation to neurocognitive and neurobehavioral findings. 2015.
10. White T, Schmidt M, Karatekin C. White matter ‘potholes’ in early-onset schizophrenia: a new approach to evaluate white matter microstructure using diffusion tensor imaging. Psychiatry Research: Neuroimaging. 2009;174(2):110-5.
11. Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, et al. Detection of blast-related traumatic brain injury in US military personnel. New England journal of medicine. 2011;364(22):2091-100.
12. Mayer AR, Bedrick EJ, Ling JM, Toulouse T, Dodd A. Methods for identifying subject‐specific abnormalities in neuroimaging data. Human brain mapping. 2014;35(11):5457-70.
13. Broglio SP, McCrea M, McAllister T, Harezlak J, Katz B, Hack D, et al. A national study on the effects of concussion in collegiate athletes and US military service academy members: the NCAA–DoD concussion assessment, research and education (CARE) consortium structure and methods. Sports medicine. 2017;47(7):1437-51.
14. Manjón JV, Coupé P, Concha L, Buades A, Collins DL, Robles M. Diffusion weighted image denoising using overcomplete local PCA. PloS one. 2013;8(9):e73021.
15. Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063-78.
16. Avants BB, Tustison N, Song G. Advanced normalization tools (ANTS). Insight j. 2009;2(365):1-35.
17. Fortin J-P, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149-70.
Figures
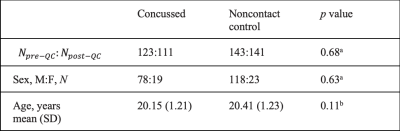
Figure 1. Study Demographics
Demographics measures at the acute concussion (i.e., 24-48 hours after injury) datapoint. aThe χ2 test and btwo sample t-test were used to test for number of datapoints (N), sex, and age differences, respectively. There were 14 datapoints failed to pass the quality control (QC). There were 14 datapoints represented multiple acute concussion for the same participants. Unique participants who passed the QC (N=97) were then tested for sex and age differences. Abbreviations: QC = quality control, SD = Standard Deviation.
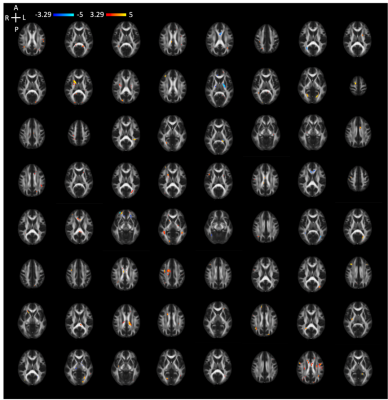
Figure 2. Extreme Z-score map of MD across 64 randomly selected concussed participants.
Extreme Z-score maps for the MD metric in 64 randomly selected concussed participants. Only 2 participants had no extreme Z-score voxels. Each individual’s extreme Z-score map was thresholded (|Z| > 3.29). Voxels with extreme Z-scores were in different white matter tracts of the brain, which illustrates spatial heterogeneity of the SRC-induced white matter abnormalities.
Figure 3. Overlapping binarized extreme Z-score maps for the DTI metrics.
Extreme Z-score maps for the MD metric in 64 representative concussed participants. Only 2 participants had no extreme Z-score voxels. Each individual’s extreme Z-score map was thresholded (|Z| > 3.29). Voxels with extreme Z-scores were in different white matter tracts of the brain, which illustrates spatial heterogeneity of the SRC-induced white matter abnormalities.