3111
GROG Gridding using modified VGG-16 CNN model for Non-Cartesian MR Image Reconstruction1Medical Image Processing Research Group (MIPRG), Department of Electrical and Computer Engineering, COMSATS University, Islamabad, Pakistan, 2National Centre of Physics (NCP), Islamabad, Pakistan
Synopsis
Keywords: Image Reconstruction, Image Reconstruction
Self-Calibrating GROG (SC-GROG) is a gridding algorithm that maps the k-space MRI data from non-Cartesian to Cartesian domain. The main limitation of SC-GROG is its computational cost to calculate the GROG weights. This paper proposes a customized deep learning framework (based on VGG-16 CNN model) to calculate the 2D-Gridding weight sets for SC-GROG. Initially, the proposed model is trained on human head images, and later fine-tuning is performed using Golden-angle radial Liver Perfusion datasets. The results show that the proposed method significantly reduces the computation time for the estimation of GROG weights while maintaining the image quality.
Introduction
Self-calibrating GRAPPA Operator Gridding1 (SC-GROG) is a gridding algorithm that shifts the k-space data from non-Cartesian space to Cartesian grid. SC-GROG1 is a computationally intensive algorithm that takes a significant computation time when it maps the NC samples in MRI data to their nearest Cartesian grid locations1. This research work aims to accelerate SC-GROG1 algorithm using a customized CNN framework (a modified VGG-162 CNN model) to reduce the computation time while maintaining quality of the reconstructed image. The proposed method pre-calculates all the possible combinations of 2D-gridding weight sets and then uses these weights to shift the NC samples for multi-channel receiver coils at the nearest Cartesian grid locations. Experiments are performed on human head datasets3 and Golden-angle radial binned Liver data set4. The results obtained from the proposed method are compared with the conventional SC-GROG1 reconstruction.Methods
The main steps of the proposed method are shown in Figure 1. Firstly, the continuously acquired Dynamic Contrast Enhanced (DCE) MRI radial data5 is divided into different frames with a set number of spokes. The spokes in each frame are sorted w.r.t the self-extracted respiratory signal5 and then divided into bins of equal no of projections (uniform binning5). The binned data is given as an input to the proposed customized VGG-162 CNN model (shown in Figure 2) to obtain the corresponding 2D-Gridding weight sets that are used to grid the radial k-space data onto Cartesian grid. Averaging of the points gridded to the same Cartesian grid location was performed1. Finally, Compressed Sensing (CS)6 is applied on this gridded data to get the solution images.In the proposed CNN model (customized VGG-162), the fully connected and softmax layers were removed2 because the under-lying problem falls under the category of a regression problem7. Initially, the proposed model was trained on human head datasets3. The training dataset was obtained by simulating the 8 receiver coil data from the single-coil T2 weighted human head images3 (acquired from 1.5T scanner with Acceleration Factor (AF=2)) using Biot-Savart Law8. These images were converted into radial k-space retrospectively using Fessler toolbox9 and used as an input to the proposed CNN model, whereas the corresponding 2D-Gridding weight sets were used as the labels for training of the proposed model. There were a total of 1050 human head images3, the first 900 images were used for training purpose and the remaining 150 images for testing.
As the Liver datasets4 have a completely different anatomy as compared to human head datasets3 (on which the proposed CNN model was initially trained), therefore, the proposed CNN model requires fine tuning for the Liver datasets4. We used a total of 104 (3D) Liver Perfusion images4, the first 66 images were used for training and the remaining were used for testing. These datasets4 were acquired from healthy volunteers on 3T scanner using free breathing Golden-angle radial acquisition with 3D stack-of stars pulse sequence4. Uniform binning5 was applied by keeping 55 spokes (chosen empirically) in each bin to minimize the motion artifacts. For training purposes, the loss function of Mean Square Error (MSE) was minimized by using the Adam Optimizer with a learning rate of 0.0001 (selected empirically) and the 40 epochs (also selected empirically).
The scripts for training and testing the proposed model were written in Python 3.9.1 using Keras library (TensorFlow was used as backend). The CPU and GPUs used in this work are (i) Intel core i7-8700 CPU having clock-frequency of 3.6GHz and 16GB RAM, and (ii) NVIDIA V100 GPU on Saturn-Cloud10.
Results
Figure 3 shows the fully-sampled reference image, the reconstruction results obtained from the conventional SC-GROG1 and from the proposed deep learning framework for the human head data set3. The PSNR and RMSE values are 197.737 dB and 3.3228e-0.8 respectively for the conventional SC-GROG1; while the PSNR and RMSE values for the proposed method are 189.184 dB and 8.8928e-0.8 respectively. This shows that both the methods provide almost similar reconstruction results; however, the conventional method offers slightly better PSNR value in this case.Figure 4 shows the fully-sampled reference image, reconstruction results of the conventional SC-GROG1 method and the proposed SC-GROG method for Golden-angle radial Liver Perfusion data set4. The PSNR and RMSE values are 62.4128 dB and 1.939e-0.1 respectively for the conventional SC-GROG1; and the PSNR and RMSE values are 62.1420 dB and 2.001e-0.1 respectively for the proposed method. This shows that both the methods provide similar reconstruction results.
The computation time for the weight estimation in conventional SC-GROG1 method is approximately 700ms in our experiments whereas the computation time in the proposed method significantly reduces to 100ms (it does not include the training time of the proposed CNN as it is performed only once). The computation time was measured by using the built-in functions in Python.
Discussion and Conclusions
This paper proposes a customized CNN model for the calculation of 2D-gridding weight-sets for SC-GROG method1 and compares the results with the conventional SC-GROG1. Initially, the proposed model is trained on human head data set3, and later fine tuning is performed for Golden-angle radial Liver Perfusion datasets4. The proposed method significantly reduces the computation time as compared to the conventional method while maintaining the reconstructed image quality.Acknowledgements
We would like to acknowledge NVIDIA Corp. Silicon Valley USA for providing us the computational resources through their partner Saturn Cloud10. A total of 400 computational hours of cloud computing resources were awarded for this work by NVIDIA Corp. Silicon Valley, USA. This resource greatly helped in reducing the training time of the proposed CNN model.References
1. N.Seiberlich, F.A. Breuer, M. Blaimer, K. Barkauskas, P.M. Jakob, M.A. Griswold, Magn. Reason. Med. 58, 1257-1265 (2007)
2. Karen Simonyan, Andrew Zisserman, Very Deep Convolutional Networks for Large-Scale Image Recognition., ICLR 2015
3. Hyun CM, Kim HP, Lee SM, Lee S, Seo JK (2018) Deep learning for undersampled MRI reconstruction. Phys Med Biol 63:135007
4. Li Feng, Otazo Ricardo. Dataset of dynamic contrast-enhanced liver MRI [Online]. Available. http://cai2r.net/resources/software.
5. Iram Shahzadi, Muhammad Faisal Siddiqui, Ibtisam Aslam, Hammad Omer, Respiratory motion compensation using data binning in dynamic contrast enhanced golden-angle radial MRI, Mag. Resin. Imaging 70 (2020) 115-125
6. Michael Lusting, David Donoho and John M. Pauly, Sparse MRI: The Application of Compressed Sensing for Rapid MR Imaging, Magnetic Resonance in Medicine 58:1182-1195 (2007)
7. Jason Browniee, Difference between Classification and Regression in Machine Learning [online]. Available: https://machinelearningmastery.com/classification-versus-regression-in-machine-learning/ (Accessed on Dec 2021)
8. Guerquin-Kern M. Matlab code for MRI simulation and reconstruction. 2012:8. http://bigwww.epfl.ch/algorithms/mri-reconstruction/PackageDoc.pdf%5Cnhttp://bigwww.epfl.ch/algorithms/mri-reconstruction/%5Cnhttp://bigwww.epfl.ch/guerquin/thesis/thesis007.html%5Cnhttp://bigwww.epfl.ch/algorithms/mri-reconstruction/PackageDoc.pdf$%5C$nh
9. Michigan Image Reconstruction Toolbox (MIRT) [online]. Available: https://web.eecs.umich.edu/~fessler/code/ (Accessed on Sep 2022)
10. Saturn Cloud Computing Resource [online]. Available: https://saturncloud.io/ (Accessed on March 2022)
Figures
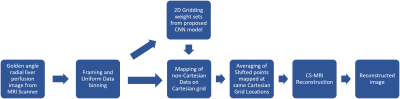
Figure 1: Main steps of the proposed method. Firstly, Golden angle radial liver perfusion data is acquired from the MRI scanner. Framing and Uniform Binning is applied to the acquired data followed by obtaining 2D GROG1 gridding weight sets using the proposed VGG-162 CNN model. The predicted weight sets were used for mapping the radial data onto Cartesian grid followed by averaging of points shifted to same Cartesian grid locations. Finally, reconstructed image is obtained by applying Compressed Sensing.
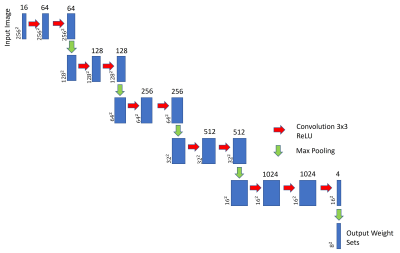
Figure 2: The proposed modified VGG-162 CNN model to predict 2D gridding weight sets for SC-GROG1 method
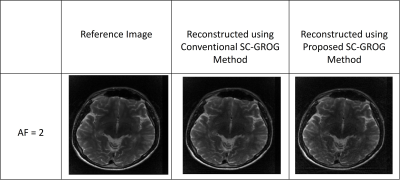
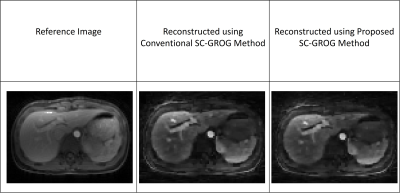
Figure 4: Reference image is shown in column 1, reconstruction results of Golden-angle radial Liver Perfusion images (having 55 projections in each bin) using conventional GROG1 and the proposed method (after applying Compressed Sensing on resultant image from both the methods) are shown in column 2 and 3 respectively.