3094
Implementation of Hyperpolarized 129Xe Accelerated MRI in Phantom and Human Lungs: Preliminary Study and Troubleshooting1Physics and Astronomy, University of Western Ontario, London, ON, Canada, 2Lawson Health Research Institute, London, ON, Canada, 3School of Biomedical Engineering, University of Western Ontario, London, ON, Canada
Synopsis
Keywords: Data Acquisition, Low-Field MRI
Accelerated MRI could significantly improve image quality of lung imaging without increasing costs, especially for low field strengths. The signal decay of a series of undersampled xenon-129 images is fitted to the Stretched-Exponential-Model to yield higher SNR. The proposed method was implemented in undersampled phantom images at low field (0.074T) and human lung images at high field (3T) using the FGRE pulse sequence with hyperpolarized 129Xe; SNR was significantly improved within the same scan duration compared to a fully-sampled image. Potential issues with the compressed-sensing reconstruction are identified and possible solutions presented to promote robustness of method.Introduction
Traditional approaches to tackle the low sensitivity of MRI, such as higher field strength and enriched contrast agents (13C),1 often incur additional material and operational costs. In the case of hyperpolarized gas lung MRI with naturally-abundant xenon-1292, this low-sensitivity issue still poses considerable problems. As the optimal field strength range for hyperpolarized 129Xe lung imaging lies between 0.1 T and 0.6 T3, accelerated MRI could improve the low sensitivity inherent of the low field regime. It has recently been shown that accelerated MRI via Compressed-Sensing, combined with fitting to the Stretched-Exponential-Model, is able to provide considerable gains in SNR without increasing total scan-duration.4,5To reduce the k-space coverage needed to construct an adequate image (and therefore the sampling time), k-space is undersampled according to high acceleration factors (AF): for example, an acceleration factor of 10 corresponds to acquiring 10% of k-space, resulting in 10x faster acquisition. If the set of images follows a predictable and known signal decay curve, the reconstruction can be fitted to this decay and improve the SNR and quality of reconstructed images. Specifically, if the SNR of this set of images is assumed to reflect the spin-density difference instead of the signal-level,4 then the signal decay of these images can be fitted to the Stretched-Exponential-Model.6-9 The feasibility of this approach was demonstrated using retroactively4 undersampled phantom and lung images, and proactively5 undersampled phantom images. Image artefacts caused by reconstruction of the accelerated k-spaces have been shown to be removable by a convolutional neural network5,10 and recent advancements in deep learning.11,12
Methods
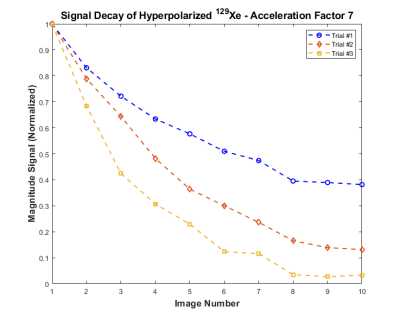
Phantom MR was performed with 45 mL of hyperpolarized (35%) 129Xe at 0.074 T using the Fast-Gradient-Recalled-Echo (FGRE) pulse sequence, modified for centric-out acquisition. This modification ensured the center of k-space was acquired first before significant T2* decay could occur; this also ensured the undersampling was done mainly around the edges of k-space, preserving important contrast information. Nine undersampled k-spaces were acquired at an acceleration factor of 7. The following parameters were used: FOV=15x15cm2, matrix=128x128, TE/TR=1ms/50ms, BW=18kHz, and Flip-Angle = 15°.As the polarization of the gas decreased with each RF pulse towards thermal equilibrium, the resulting signal decay can represent the decreasing gas density of 129Xe in lungs after each wash-out breath13,14. The signal of each image was plotted as a function of its image number and fitted to the Stretched-Exponential-Model using the Abascal method.4,7
A 3-stage U-Net15 was previously developed5 to remove or minimize reconstruction artefacts on 1H phantom reconstructions, and was directly applied to 129Xe phantom data.
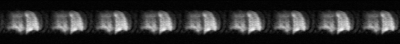
One healthy-volunteer with written informed consent, provided to an ethics-board-approved study protocol, underwent spirometry and 129Xe MRI scanning. Nine undersampled (AF=7) 129Xe human-lung images per slice were acquired at 3.0T (MR750, GEHC) using whole-body-clinical-gradients and a commercial, xenon-quadrature-flex human RF-coil16 (MR Solutions). A centric-out interleaved 2D FGRE sequence was used for the seven 30 mm coronal-slices (TE/TR = 2.1 ms/51 ms, matrix size=128x128, FOV=40x40cm2, constant-flip-angle = 4o, and BW = 31.5 kHz). All images were acquired in breath-hold (<7 sec) after inspiration of 1.0L of gas (129Xe/4He mixture, 30%/70%) from functional-residual-capacity, and the total scan time was 1 second per slice. Hyperpolarized 129Xe gas (polarization=33%) was obtained from a turn-key polarizer system (Polarean-9820 129Xe polarizer)17.
Results
The signal decay of the hyperpolarized 129Xe in phantom is shown in Fig.1. Fig.2 depicts the phantom data previously acquired5 and the filtered 129Xe data (both AF=7). Accelerated human lung images from the third slice are shown in Fig.3, with consistent signal level.Discussion and Conclusion
The phantom reconstructions show a clear increase in signal from the hyperpolarized 129Xe, but due to the presence of strong low-frequency RF interference the reconstruction broke down and failed, as the algorithm relies heavily on reproducible patterns in the raw data. Although the cause of this interference (Fig.4) is still under investigation, this issue highlights a weak link in the reconstruction algorithm which could be problematic for low field lung imaging: the reconstruction must be adjustable and adaptable to account for possible differences in hardware, as well as k-space regridding and sequence design from different manufacturers. As such, the artefact removal neural network was unable to remove artefacts in the AF=7 phantom reconstruction.Despite using an acceleration factor of 7, the lung images show adequate and consistent SNR across the slice and identifiable features. The steady signal level was due to different sequence design for FGRE for the 3T GE scanner used: instead of acquiring each line of k-space in sequence to form an image before moving on to the next, the first line of every image is acquired before moving on to the second line. The absence of the characteristic signal decay hinders the reconstruction as it provides less predictable information about the raw dataset, but could be remedied by introducing the averaging pattern used in previous studies4,5.
As this is the first demonstration of this method in phantom and human lungs whilst using hyperpolarized 129Xe, these obstacles are opportunities to improve the method and reconstruction algorithm.
Acknowledgements
The authors would like to thank the research/financial supports received from NSERC Discovery-Grant (R5942A04).References
1 Thind, K. et al. Detection of radiation-induced lung injury using hyperpolarized (13)C magnetic resonance spectroscopy and imaging. Magn Reson Med 70, 601-609, doi:10.1002/mrm.24525 (2013).
2 Stewart, N. J., Norquay, G., Griffiths, P. D. & Wild, J. M. Feasibility of human lung ventilation imaging using highly polarized naturally abundant xenon and optimized three-dimensional steady-state free precession. Magn Reson Med 74, 346-352, doi:10.1002/mrm.25732 (2015).
3 Parra-Robles, J., Cross, A. R. & Santyr, G. E. Theoretical signal-to-noise ratio and spatial resolution dependence on the magnetic field strength for hyperpolarized noble gas magnetic resonance imaging of human lungs. Med Phys 32, 221-229, doi:10.1118/1.1833593 (2005).
4 Perron, S., Fox, M. S., Serrai, H. & Ouriadov, A. Feasibility of a Novel Sampling/Reconstruction Method Ensuring a SNR Benefit Over the Traditional Sampling Approach. Proceedings of the 30th Annual Meeting of ISMRM, Vancouver, Canada (2021).
5 Perron, S., Fox, M. S. & Ouriadov, A. Improvements of Image Quality of 1H and 129Xe MRI by Using an Advanced Acquisition and Reconstruction Method Coupled with Deep Learning. Proceedings of the 31st Annual Meeting of ISMRM, London, UK. (2022).
6 Westcott, A., Guo, F., Parraga, G. & Ouriadov, A. Rapid single-breath hyperpolarized noble gas MRI-based biomarkers of airspace enlargement. J Magn Reson Imaging 49, 1713-1722, doi:10.1002/jmri.26574 (2019).
7 Abascal, J., Desco, M. & Parra-Robles, J. Incorporation of Prior Knowledge of Signal Behavior Into the Reconstruction to Accelerate the Acquisition of Diffusion MRI Data. IEEE Trans Med Imaging 37, 547-556, doi:10.1109/TMI.2017.2765281 (2018).
8 Berberan-Santos, M. N., Bodunov, E. N. & Valeur, B. Mathematical functions for the analysis of luminescence decays with underlying distributions 1. Kohlrausch decay function (stretched exponential). Chemical Physics 315, 171-182, doi:10.1016/j.chemphys.2005.04.006 (2005).
9 Parra-Robles, J., Marshall, H. & Wild, J. M. Characterization of 3He Diffusion in Lungs using a Stretched Exponential Model [abstract]. ISMRM 21st Annual Meeting, 0820 (2013).
10 Lee, D., Yoo, J. & Ye, J. C. Deep artifact learning for compressed sensing and parallel MRI. Magnetic Resonance in Medicine (2017).
11 Yang, Y., Sun, J., Li, H. & Xu, Z. ADMM-CSNet: A Deep Learning Approach for Image Compressive Sensing. IEEE Transactions on Pattern Analysis and Machine Intelligence PP, 1-1, doi:10.1109/TPAMI.2018.2883941 (2018).
12 Hammernik, K. et al. Learning a variational network for reconstruction of accelerated MRI data. Magnetic Resonance in Medicine 79, 3055-3071, doi:https://doi.org/10.1002/mrm.26977 (2018).
13 Ouriadov, A. V. et al. In vivo regional ventilation mapping using fluorinated gas MRI with an x-centric FGRE method. Magn Reson Med 74, 550-557, doi:10.1002/mrm.25406 (2015).
14 Santyr, G. E., Lam, W. W. & Ouriadov, A. Rapid and efficient mapping of regional ventilation in the rat lung using hyperpolarized 3He with Flip Angle Variation for Offset of RF and Relaxation (FAVOR). Magn Reson Med 59, 1304-1310, doi:10.1002/mrm.21582 (2008).
15 Ronneberger, O., Fischer, P. & Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. ArXiv abs/1505.04597 (2015).
16 Kaushik, S. S. et al. Single-breath clinical imaging of hyperpolarized (129)Xe in the airspaces, barrier, and red blood cells using an interleaved 3D radial 1-point Dixon acquisition. Magn Reson Med 75, 1434-1443, doi:10.1002/mrm.25675 (2016).
17 Kaushik, S. S. et al. Diffusion-weighted hyperpolarized 129Xe MRI in healthy volunteers and subjects with chronic obstructive pulmonary disease. Magn Reson Med 65, 1154-1165, doi:10.1002/mrm.22697 (2011).
Figures