3080
High resolution 4D-cine MRI of pulsatile aneurysmal motion with radial acquisition at 7T
Thai Akasaka1, Koji Fujimoto2, Martijn Cloos3, Tomohisa Okada1, Shinichi Urayama1, and Isa Tadashi1
1Human Brain Research Center, Kyoto University, Kyoto, Japan, 2Real World Data Research and Development, Kyoto University, Kyoto, Japan, 3University of Queensland, Brisbane, Australia
1Human Brain Research Center, Kyoto University, Kyoto, Japan, 2Real World Data Research and Development, Kyoto University, Kyoto, Japan, 3University of Queensland, Brisbane, Australia
Synopsis
Keywords: Image Reconstruction, Image Reconstruction
The pulsation of intracranial aneurysms (IAs) is a novel risk factor of rupture. We aim to visualize the pulsation of IAs with 7T MRI using GRASP, a relatively novel MRI technique that provides high spatial and temporal resolution. Using thsis method, we were able to depict the pulsation of an aneurysm phantom as a 4D-cine movie. This technique may be useful in predicting the probability of IA rupture more accurately.
Introduction
The mortality rate from ruptured intracranial aneurysms (IA) is as high as 35-50% and nearly half of the survivors suffer from permanent disability (1). Prophylactic procedures such as clipping and coiling are effective, but they carry the risk of poor neurological outcome of up to 10%, well above the mean 5-year risk of rupture, 3.4% (2). Moreover, retrospective studies have identified a much higher frequency of small, ruptured IAs than expected from previous natural history studies. This calls for more accurate rupture risk predictors. Pulsation of IAs has been reported as a novel candidate to predict rupture, but no standardized evaluation method is yet available (3). In this study we aim to visualize the pulsation of IAs by leveraging the high SNR of 7T MRI with GRASP (Golden-Angle Radial Sparse Parallel), a relatively novel MRI technique that provides high spatial and retrospectively configurable temporal resolution.Methods
To test the newly developed MRI sequence and reconstruction algorithm for GRASP reconstruction, a pulsating phantom was first constructed by combining a rigid tubular structure with holes created by a digital light processing 3D printer and an ultra-flexible rubber tube. The phantom was housed in an MRI-compatible acrylic case, which was connected to a pulsatile flow pump and controller (Alpha Flow SP-1, Alpha FC PR-1, Fuyo Corporation, Japan) to simulate the pulsation of an aneurysm (Figure 1). The pulsating phantom was scanned with an in-house radial gradient echo (GRE) 3D MRI sequence (TR/TE = 13/6.6ms, FA = 12°, spokes = 1000, spatial resolution = 0.5 x 0.5 x 0.5mm, field of view = 160 x 160 x 11 mm, scan time = 4m46s, flow compensation = ON) on a 7T MRI scanner (MAGNETOM 7T, Siemens Healthineers, Germany) with a 32-channel receiver head coil (Nova Medical, USA). The acquired data was synchronized with the ECG signal output from the pulsatile pump (set to 21 beats per minutes) and reconstructed into 16 time phases per pulsation using our image reconstruction algorithm.Three healthy volunteers (21, 21 and 23 yr. old male) were recruited for a whole brain scan with the same MRI sequence as above (TR/TE = 10/5.5ms, FA = 15°, spokes = 512, spatial resolution = 0.3 x 0.3 x 0.6mm, field of view = 152 x 152 x 72 mm, scan time = 10m16s, flow compensation = ON). MR angiography (MRA) images were constructed from the scanned images using the maximum intensity projection (MIP) method.
Results
The aneurysm phantom was able to steadily pulsate at a rate of 21 to 60 beats per minute, for an arbitrary length of time (Figure 1). The diameters of the aneurysms were 4.0mm, 3.5mm, 3.0mm and 2.5mm and their respective amplitudes of wall motion were approximately 2.0mm, 1.0mm, 0.5mm and nil. When the largest aneurysm of the phantom was scanned with our in-house MRI sequence, pulsation was observed in the first 6 time phases of the 16 time phases (Figure 2). Three MRA MIP images of 3 healthy volunteers’ intracranial arteries including the perforating arteries were successfully depicted (Figure 3).Discussion
Stam et al. has recently reviewed imaging techniques and quantitative measurements for dynamic imaging of cerebral aneurysm pulsations (4). Most of the recent research used contrast-enhanced 4D-CT with resolution up to 0.3 x 0.3 x 0.5 mm3 (5), but frequent irradiation is inevitable for longitudinal observations. Kleinloog et al. used Turbo-field echo MRA at 7T to measure aneurysm volume pulsation as a potential predictor of intracranial aneurysm rupture in an 0.6-mm, isotropic resolution (6). For small aneurysms, higher resolution is preferable, but longer scan time may blur the images, especially when Cartesian sampling is employed. Therefore, high resolution radial scan MRA was implemented in this study. We successfully visualized the pulsation of phantom wall motion of the aneurysm model up to 0.5mm synchronized with simulated cardiac pulsation, which is comparable to the contrast-enhanced 4D-CT scan. Although, high-resolution 4D-MRA takes a long scan time, subject motion can be suppressed due to the advantage of the radial scan.Conclusion
The radial GRE sequence and GRASP reconstruction at 7T was successfully able to depict the pulsation of an aneurysm phantom as a 4D-cine movie. This technique may be used as a novel tool to depict the pulsation of IAs and more accurately predict the probability of their rupture.Acknowledgements
Special thanks to Mr. Yuta Urushibata, Siemens Healthcare K.K.References
- Hackenberg, K. A., Hänggi, D., & Etminan, N. (2018). Unruptured intracranial aneurysms: contemporary data and management. Stroke, 49(9), 2268-2275
- Wiebers, David O., and International Study of Unruptured Intracranial Aneurysms Investigators. "Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment." The Lancet 362.9378 (2003): 103-110.
- Mocco, J., et al. "Aneurysm morphology and prediction of rupture: an international study of unruptured intracranial aneurysms analysis." Neurosurgery 82.4 (2018): 491-496.
- Stam LB, Aquarius R, de Jong GA, et al. A review on imaging techniques and quantitative measurements for dynamic imaging of cerebral aneurysm pulsations. Sci Rep. 2021;11(1):2175.2.
- Dissaux B, Ognard J, El Aouni MC, et al. Volume variation may be a relevant metric in the study of aneurysm pulsatility: A study using ECG-gated 4D-CTA (PULSAN). J. Neurointerv. Surg. 2020; 12(6), 632–636.3.
- Kleinloog R, Zwanenburg JJM, Schermers B, et al. Quantification of Intracranial Aneurysm Volume Pulsation with 7T MRI. Am J Neuroradiol. 2018 Apr;39(4):713-719.
Figures
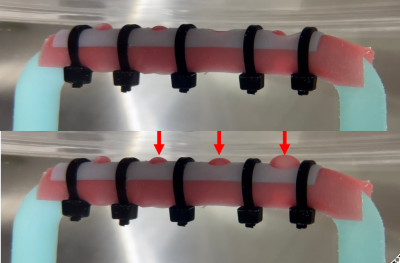
Figure 1. The pulsating phantom made of a rigid tubular structure with holes created by a digital light processing 3D printer. The aneurysm portion covered with ultra-flexible rubber sheet was covered with a lid with open holes then tightly secured with cable ties. The pulsation is depicted (red arrows) in the lower picture.
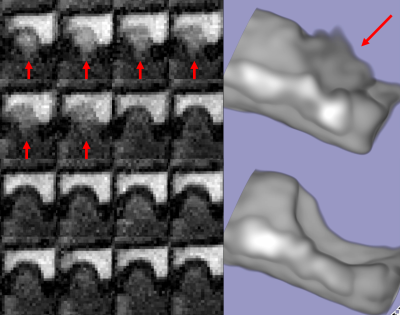
Figure 2. 2D coronal view of the the 16 time phases of the pulsating aneurysm with a diameter of 4mm (left image) and its 3D rendering (right image). The wall movement of the aneurysm is observed in the first 6 phases (red arrows).
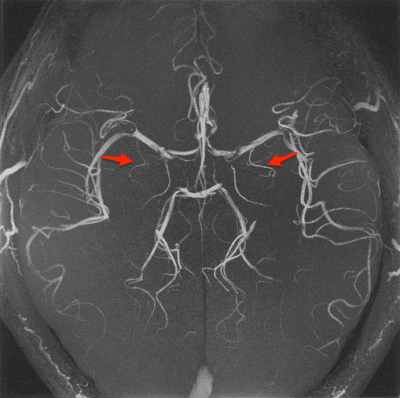
Figure 3. A maximum intensity projection image of the brain of a healthy volunteer. The perforating arteries are depicted in red arrows.
DOI: https://doi.org/10.58530/2023/3080