3070
Carotid arterial stiffening is associated with reduced downstream territorial perfusion of internal carotid artery in elderly adults1Radiology, Northwestern University, Chicago, IL, United States, 2Biomedical Engineering, Northwestern University, Evanston, IL, United States, 3Neurology, University of Southern California, Los Angeles, CA, United States
Synopsis
Keywords: Blood vessels, Aging
Arterial stiffness is an important risk marker for poor brain aging, vascular disease, and dementia. Greater arterial stiffness leads to the transmission of excessive pulsations from the greater vessels into the downstream capillary and tissue causing microvascular dysfunction. However, previous studies have mainly focused on central or peripheral pulse wave velocity assessment. The present study has demonstrated a significant association of the PWV of the feeding arteries to the brain and its downstream territorial perfusion assessed by using two new MRI techniques, which could provide valuable insight into the neurovascular pathology of aging and brain dysfunction.Introduction
A growing body of research indicates that arterial stiffness is an important risk marker for poor brain aging, vascular disease, and dementia1. Greater arterial stiffness leads to the transmission of excessive pulsations from greater vessels into the downstream capillary and tissue causing microvascular dysfunction. Arterial stiffness is commonly assessed by measuring pulse wave velocity (PWV). However, existing approaches mainly measure the central and/or peripheral PWV2. The association between the arterial stiffness of feeding arteries in the brain, such as the internal carotid artery, and downstream cerebral territorial perfusion has not been fully elucidated. A previous study has introduced an oblique-sagittal phase-contrast MRI (OS PC-MRI) method for directly assessing carotid PWV (cPWV) between the common carotid artery and internal carotid artery (CCA-ICA)3. The goal of this study is to investigate the association between carotid arterial stiffness measured using OS PC-MRI and downstream territorial perfusion generated by random vessel-encoded ASL4 individually in a cohort of elderly adults.Methods
MR Imaging protocol: All experiments were carried out on a Siemens 3T Prisma MRI scanner (Magneton Prisma, Siemens Healthcare, Erlangen, Germany) using a 20-channel head coil. 25 elderly participants ( 14 female, 72+-7.38years ) were enrolled in this study after providing informed consent. A quick time-of-flight (TOF) MR angiography scan was performed to localize carotid arteries including CCA and ICA. ECG retrospectively gated OS-PC MRI was performed on each subject to cover the most CCA-ICA segment with the following imaging parameters: resolution=1x1x1mm3, Venc=80cm/s, TE/TR=4.32/14.22ms, flip angle=10°, real temporal resolution=14.22ms, number of phases=70-90, scan time is 1 to 2min depending on the heart rate of each participant. Random VE-ASL data was collected to generate perfusion territorial maps with the imaging parameters: FOV= 220×220 mm2, matrix size=64×64, TE/TR=22/3500ms, 16 slices with slice thickness of 6mm, labeling duration=1500ms, post-labeling delay (PLD)=1500ms, 60 pairs of encoding steps with random orientation, phase and wavelength were acquired with two additional pairs of global label/control. Multi-delay ASL was also performed with imaging parameters: FOV=220x220mm2; resolution=96x96; TE/TR=37/4100ms; 54 slices with slice thickness of 2.5mm; lableing duration=1500ms, PLDs=500ms/1300ms/1500ms/1800ms/2300ms. Magnetization Prepared Rapid Gradient Echo (MPRAGE) was acquired for generating the gray matter mask.Data analysis: The velocity waveform across a cardiac cycle was measured at each axial position along CCA-ICA. The time-to-feet method was used to calculate the transit time along the vessel. cPWV was calculated as the inverse slope of the line fitted to the transit-time versus distance along the vessel. Perfusion territorial mapping and corresponding feeding arteries (left ICA, right ICA, and vertebral artery) were generated simultaneously from rVE-ASL data4. Left and right ICA territorial masks were generated from each subject. CBF and ATT maps were calculated from the multi-delay ASL data. Mean gray matter CBF and ATT values were extracted from each subject. Mean ICA territorial CBF (tCBF) and ATT (tATT) values were calculated from the multi-delay ASL-generated CBF and ATT maps within the ICA territory mask generated from rVE-ASL from the same side as that of cPWV. Two rVE-ASL data showed bad quality due to the head motion and artifacts. The perfusion territories from the two subjects were acquired from the standard VE-ASL instead. The correlations between cPWV vs. CBF and ATT were calculated across subjects using Pearson correlation coefficients.
Results
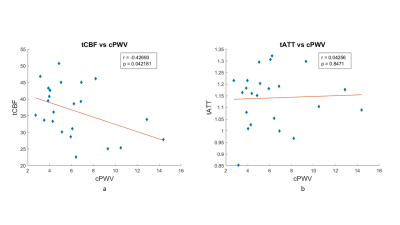
Figures 1 a and b show the scatter plots of mean gray matter CBF vs. cPWV and mean gray matter ATT vs. cPWV, respectively. Mean gray matter CBF significantly reduced with elevated cPWV (r=-0.42; p=0.035), whereas there were no changes in ATT as cPWV increased (r=-0.04; p=0.83).Figures 2 a and b show the scatter plots of ipsilateral ICA territorial CBF vs. cPWV and ICA territorial ATT vs. cPWV, respectively. Similar to the findings from gray matter, there was a significant association between cPWV and ICA territorial CBF (r=-0.43; p=0.042) whereas no association was observed between cPWV and territorial ATT (r=0.04; p=0.85). No significance was observed between cPWV vs. the contralateral ICA territorial CBF and ATT. These findings indicate that elevated cPWV was accompanied by the reduction in its downstream directly associated territorial CBF.
Discussion & Conclusion
This study is the first to directly study the relationship between arterial stiffness of the feeding arteries in the brain and perfusion of its downstream vascular territory from individuals. This study shows several advantages, compared to previous research. First, OS PC-MRI was applied to directly access the arterial stiffness of feeding arteries in the brain, instead of accessing the central or peripheral arterial stiffness. Second, variations in cerebral vascular territories have been commonly observed, especially in elderly subjects. In this work, we extracted the accurate ICA territory from individuals using rVE-ASL instead of using a vascular atlas. This study provides a direct evidence between carotid arterial stiffness and its downstream carotid territorial CBF, which could enhance our understanding of the link between macrovascular and microvascular dysfunction as well as the underlying neurovascular pathology of aging and cognitive decline.Acknowledgements
This work is supported by grants of NIH R01NS118019, RF1AG072490, and BrightFocus Foundation A20201411S.References
1. Parittotokkaporn S, de Castro D, Lowe A, Pylypchuk R. Carotid Pulse Wave Analysis: Future Direction of Hemodynamic and Cardiovascular Risk Assessment. JMA J. 2021;4(2):119-128. doi:10.31662/jmaj.2020-0108
2. Meyer ML, Palta P, Tanaka H, Deal JA, Wright J, Knopman DS, Griswold ME, Mosley TH, Heiss G. Association of central arterial stiffness and pressure pulsatility with mild cognitive impairment and dementia: the Atherosclerosis Risk in Communities Study-Neurocognitive Study (ARIC-NCS). Journal of Alzheimer's Disease. 2017 Jan 1;57(1):195-204.
3. Heidari Pahlavian S, Cen SY, Bi X, Wang DJJ, Chui HC, Yan L. Assessment of carotid stiffness by measuring carotid pulse wave velocity using a single-slice oblique-sagittal phase-contrast MRI. Magn Reson Med. 2021;86(1):442-455. doi:10.1002/mrm.28677
4. Wong EC, Guo J. Blind detection of vascular sources and territories using random vessel encoded arterial spin labeling. Magnetic Resonance Materials in Physics, Biology and Medicine. 2012 Apr;25(2):95-101.