3069
Automated modeling and morphologic analysis of deep medullary veins at 7T MRI in patients with cognitive impairment1State Key Laboratory of Brain and Cognitive Science, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China, Beijing, China, 2University of Chinese Academy of Sciences, Beijing, China, Beijing, China, 3The Innovation Center of Excellence on Brain Science, Chinese Academy of Sciences, Beijing, China, Beijing, China, 4Department of Neurology, the Sixth Medical Center, Chinese PLA General Hospital, Beijing, China, 5Siemens Shenzhen Magnetic Resonance Ltd., Shenzhen, China, Shenzhen, China, 6Institute of Artificial Intelligence, Hefei Comprehensive National Science Center, Hefei, China, Hefei, China
Synopsis
Keywords: Machine Learning/Artificial Intelligence, Modelling
Deep medullary veins (DMVs) support cerebral venous drainage. They may display abnormal changes in patients with cognitive impairment. They can be visualized by multi-echo gradient echo imaging at 7T. This study proposed a segmentation and tracking method based on deep learning and shortest-path optimization. It automatically quantified the morphologic parameters of DMVs from the vascular model. These characteristics of DMVs correlated with the patients’ cognitive scores, and might reflect the pathology of vascular lesions in cognitive impairment.Introduction
High-resolution multi-echo gradient echo (GRE) sequence at 7T allows us to visualize the deep medullary veins (DMVs) comprehensively 1. The pathologic changes in DMVs, such as increased tortuosity and reduced vascular density, were reported to be closely related to cognitive decline and dementia 2 3. However, the modeling and analysis of DMVs were challenging due to the limited contrast-to-noise ratio of GRE and small calibers of DMVs. We proposed a multi-input Res-Net and a shortest-path algorithm for the robust segmentation and modeling of DMVs in patients with cognitive impairment. 4Methods
The study included 17 patients with cognitive impairment and 8 healthy controls. The cognitive function was assessed with the Mini-Mental State Examination (MMSE) and the Vascular Dementia Assessment Scale cognitive subscale (VaDAS-Cog). The images were acquired on a 7T research MRI system (Siemens, Erlangen, Germany) equipped with a 32-channel head coil (Nova Medical). The main parameters for the multi-echo 3-dimensional (3D) GRE sequence were as follows: resolution = 0.4 × 0.4 × 0.8 mm3; matrix = 462 × 500 × 128; TR = 36 ms; TE = 5.98/12.14/18.3/24.46/30.62 ms; FA = 14°. The T1w-MPRAGE was acquired with 0.70-mm resolution as a structural reference.The N4 bias field correction (ANTs) and the brain extraction were sequentially applied in the preprocessing stage. The position of DMVs was determined using the expanded ventricular surfaces of both ventricles, as previously described 5.
The Res-Net was used to accept multiple inputs in the segmentation stage. Figure 1 represents the architecture. The training employed 5000 positive and 20,000 negative points from 8 participants. Inputs for every point were as follows:
a. Small patches with a scale of 15 × 15 × 15 from both the magnitude and phase images of 5 echoes.
b. Big patches with a scale of 64 × 64 × 64 from the magnitude image of the second echo.
c. Hessian matrix calculated in the region with a scale of 9 × 9 × 9.
The endpoint of each step was selected from the centers of the end faces on propagating cylinders in the tracking stage. The energy loss function was used to tolerate signal perturbations containing:
a. Sum of the signal intensities in the cylinder.
b. Distance to the end point.
c. Arccosine of the angle at which the cylinder deviated from the current propagating vector.
d. Arccosine of the angle contained by the cylinder and the initial vector.
The geometric parameters of DMVs and the cognitive assessment scores were compared between patients and controls using the independent samples t test. Pearson correlation coefficient between geometric parameters and cognitive impairment was calculated.
Results
Figure 2 shows the reconstructed 3D vascular model of DMVs. We conducted ablation studies to test each component in multi-input Res-Net, as shown in Table 1.Table 2 summarizes the quantitative morphologic characteristics of DMVs. Patients with cognitive impairment had fewer DMVs (mean difference = –60.710 ± 21.810, P = 0.011) and higher curvature (mean difference = 0.121 ± 0.022, P < 0.0001), lower MMSE (mean difference = –3.071 ± 1.443, P = 0.047), and higher VaDAS-Cog scores (mean difference = 4.332 ± 1.992, P = 0.036). The numbers of DMVs and MMSE scores were moderately positively correlated (r = 0.437, P = 0.037). The DMV curvature and MMSE scores were negatively correlated (r = –0.426, P = 0.042). No correlation was observed between other morphologic parameters and cognitive scores. Figure 3 and Table 2 present the statistical analysis findings.
Discussion
The proposed method provided reliable 3D models of DMVs based on 7T multi-echo GRE. Finer segmentation results were obtained using the multiple inputs of magnitude and phase images. The length, diameter, and curvature of DMVs were obtained without intensive labor and subjective variability. The method might help examine the morphology of DMVs.The method reported a reduced number and increased tortuosity of DMVs in patients with cognitive impairment, reflecting that the metrics derived from the method could reflect the course of the disease.
The method had certain limitations. First, high-resolution multi-echo GRE was sensitive to motion artifacts mimicking tiny veins. Second, a higher concentration of deoxyhemoglobin in certain diseases might improve the visibility of veins. It makes the interpretation to the morphological changes of DMVs more complicated.
Conclusions
We proposed a Res-Net-based approach to determine the geometric parameters of DMVs from multi-echo GRE images and examine the morphologic changes in DMVs in patients with cognitive impairment. It might help identify the role of DMVs in disease development.Acknowledgements
This study has received funding from the National Natural Science Foundation of China (82271985, 82001804, 8191101305), the capital health research and development of special (2020-2-5115), the Ministry of Science and Technology of China (2022ZD0211901, 2019YFA0707103), the Natural Science Foundation of Beijing Municipality (7191003).
References
1 Mucke, J. et al. Asymmetry of deep medullary veins on susceptibility weighted MRI in patients with acute MCA stroke is associated with poor outcome. PloS one 10, e0120801 (2015).
2 Bouvy, W. H. et al. Abnormalities of cerebral deep medullary veins on 7 Tesla MRI in amnestic mild cognitive impairment and early Alzheimer’s disease: a pilot study. Journal of Alzheimer's Disease 57, 705-710 (2017).
3 Thore, C. R. et al. Morphometric analysis of arteriolar tortuosity in human cerebral white matter of preterm, young, and aged subjects. Journal of neuropathology and experimental neurology 66, 337-345 (2007).
4 Koçak, T., Aydın, M. & Kiraz, B. in 2021 6th International Conference on Computer Science and Engineering (UBMK). 751-756 (IEEE).
5 Kuijf, H. J. et al. Quantification of deep medullary veins at 7 T brain MRI. European radiology 26, 3412-3418 (2016).
Figures
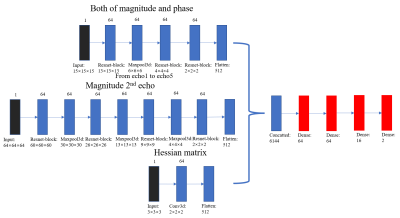
Figure 1. Architecture of the multi-input Res-Net for segmentation.
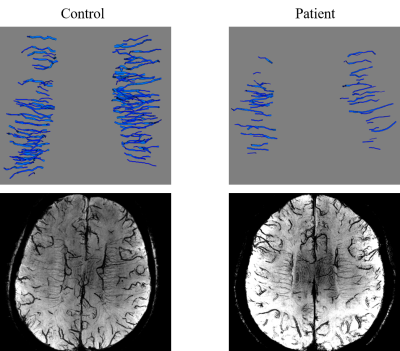
Figure 2. Three-dimensional models and the maximal intensity projection of a healthy control and a patient with cognitive impairment.
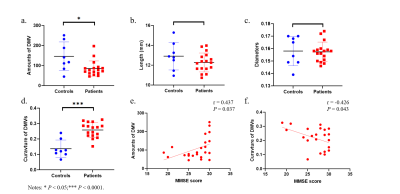
Figure 3. Statistical analysis of healthy controls versus patients with cognitive impairment. (a) Numbers of DMVs. (b)Lengths of DMVs. (c) Diameters of DMVs. (d) Curvatures of DMVs. (e) Correlation between MMSE score and numbers of DMVs. (f) Correlation between MMSE score and curvatures of DMVs.
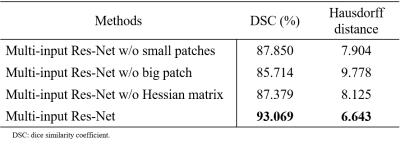
Table 1. Performance comparison with multiple inputs in ablation experiments.
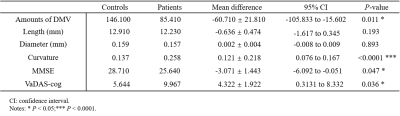
Table 2. Morphologic metrics of DMVs and cognitive scores in healthy controls and patients with cognitive impairment.