3052
Studying the Impact of Diabetes on the Pathogenesis of Alzheimer’s Disease1Biochemistry and Molecular Medicine, University of California, Davis, CA, United States, 2Nutrition, University of California, Davis, CA, United States, 3Radiology, Stanford University, Stanford, CA, United States, 4Center for Genomic Pathology Laboratory, University of California, Davis, CA, United States
Synopsis
Keywords: Alzheimer's Disease, Neurodegeneration, Hyperpolarized C13 MRI/MRS Metabolism
How type 2 diabetes (T2D) increases the risk of Alzheimer’s disease (AD) frames the central research question. The study has developed a rat model that exhibits both T2D and AD (T2D-AD +/-). These hemizygous animals contain the genes APPswe and PS1ΔE9 and display amyloid burden as early as 6 mos of age. Standard Tg344-AD +/- model shows amyloid plaque at 9 mos of age.
AD impairs cognitive performance but brain metabolism as observed with Barnes Maze test and dynamic nuclear polarization experiments.
MRS/MRI and physiological studies can now use the animal model to interrogate how insulin insensitivity accelerates AD onset/progression.Introduction
With aging, cognitive function declines sharply: 5-8% of all people between ages 65-74, 20% between ages of 75-84, and 30-47% over ages of 85 suffer from dementia, such as the common Alzheimer’s Disease (AD). Research has detected amyloid plaques and neurofibrillary tangles in AD brain and has implicated plaques and tangles in impairing and eventually destroying neurons. Despite the well-corroborated characterization, the biochemical mechanisms underlying AD pathogenesis remain unclear. To date, no intervention exists to prevent or slow AD onset and progression.Epidemiological studies have cast a novel perspective on AD: Type II diabetes (T2D) increases substantially the risk for AD. Since T2D afflicting 8.3% of the US population will trend higher in the future, the impending rise in the AD scourge should sound a public health alarm. How insulin insensitivity alters brain function poses fundamental questions in AD and in diabetes research.
The present project has attempted to clarify the question by developing a rat model that displays both T2D onset/progression with age and obesity and the AD phenotype. Such a model will facilitate the investigation into the mechanism underlying the T2D risk in AD pathogenesis.
Methods
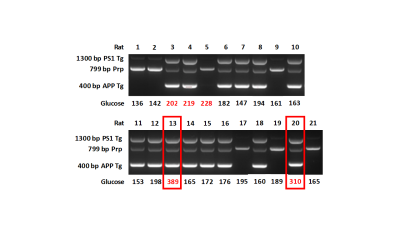
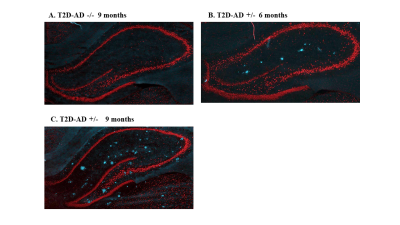
Cross breeding of the established UCD_T2DM rat model with the Tg344AD +/- rat model that contains the hemizygous AD genes (APPswe and PS1ΔE9) produced a T2D-AD +/- with the transgenes in filial generation 91,2. At 9 mos of age, both the T2D-AD +/- and Tg344AD +/- brain displayed an abundance of amyloid plaque as detected with propidium Iodide (PI) and F1-Fluoro-2,5-bis[(E)-3-carboxy-4-hydroxystyryl] benzene solution (FSB). At 6 mos, however, only T2D-AD +/- brain showed an amyloid burden. Without the transgenes neither the T2D-AD -/- and Tg344AD -/- brain showed any amyloid plaque. Wild type rat, T2D-AD -/-, and Tg344AD -/- did not spontaneously develop any amyloid at all ages. Experiments confirmed that the Tg344AD +/- transgenes intercalated into identical gene sites in the T2D-AD +/- animal.Results
Animals with the AD transgenes display reduced cognitive performance in the Barnes Maze but do not appear to alter other physiological attributes (e.g. body weight, onset of T2D). Nevertheless, the expression of the AD transgenes impairs oxidative brain metabolism as observed with dynamic nuclear polarization (DNP) magnetic resonance (MR) experiments. In the DNP experiments using1-13C Pyr as the precursor, T2D-AD -/- brain shows a marked decrease in bicarbonate/lac (Bic/Lac) ratio relative to the control (CRL) brain. The Bic/Lac ratio reflects pyruvate dehydrogenase (PDH) activity. T2D rat brain with the AD transgene (T2D-AD +/-) decreases further the Bic/Lac ratio.Conclusion
A rat model can now facilitate the investigation into the mechanism underlying the increased AD risk with T2D. In contrast to precepts, insulin insensitivity appears to alter brain metabolism in T2D and in AD.Acknowledgements
We gratefully acknowledge funding support from California Department of Public Health 18-10923 and the assistance of Lauren Andrews, Angelica Michelle Bachman, Angela Dejesus, Julia Dejesus, Meera Kohli, Rosalinda Moreno, Katelyn Nielsen, Julianna Porter, and Amy Vansdadia.References
1. Cohen, R., Rezai-Zadeh, K. Weitz, T. et al. A Transgenic Alzheimer Rat with Plaques, Tau Pathology, Behavioral Impairment, Oligomeric Aβ, and Frank Neuronal Loss. J. NeuroSci, 2013; 33(15): 6245–6256. doi: 10.1523/JNEUROSCI.3672-12.2013.
2. Cummings, B., Digitale, E., Stanhope, K. et al. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am. J. Physiol. 2008; 295 (6): R1782-93. doi: 10.1152/ajpregu.90635.2008.
Figures