3050
Prediction of amnestic mild cognitive impairment using radiomics-clinical model based on 3D-T1WI imaging1the Nanxishan Hospital, Guangxi Zhuang Autonomous Region, guilin, China, 2Graduate School of Guilin Medical University, guilin, China, 3Philips Healthcare, Guangzhou, China
Synopsis
Keywords: Alzheimer's Disease, Radiomics
Mild cognitive impairment (MCI) is a transitional state between normal aging and dementia disorders, especially Alzheimer's disease (AD). In this study, we aimed to develop a radiomics model based on 3D-T1W imaging to distinguish amnestic mild cognitive impairment (aMCI) patients from the normal elderly population by measuring changes in the frontal lobe. And the result shows that a quantitative nomogram based on clinical feature and radiomic features of MRI imaging can be used to distinguish aMCI and CN with excellent predictive ability, which can be served as a potential decision support tool to assist clinicians in Screening community aMCI population.
Introduction
Mild cognitive impairment (MCI) is a transitional state between normal aging and dementia disorders, especially Alzheimer's disease (AD) [1] and it is very important to find aMCI in the early stage. Previous studies on DWI-MRI, structure MRI (sMRI) and functional MRI (fMRI)have shown that the frontal lobe has been damaged during the aMCI and AD, specifically the frontal-subcortical circuits and the integrity of the white matter of the brain [2-5]. We aimed to develop a radiomics model based on 3D-T1W imaging to distinguish amnestic mild cognitive impairment (aMCI) patients from the normal elderly population by measuring changes in the frontal lobe.Methods
126 patients with aMCI and 174 matched healthy volunteers were recruited in this study. All participants underwent routine 3D-T1WI MRI examination on a 3.0 T MRI system (Ingenia CX, Philips Healthcare, Best, The Netherlands). Then all participants were randomly divided into a training set (n=242, aMCI: 102, healthy volunteers:140) and a testing set (n = 58, aMCI:24, healthy volunteers:34). 1138 features of frontal lobe were extracted based on T1WI images. The least absolute shrinkage and selection operator (LASSO) were used to reduce the dimension of the features and establish a radiomics signature model. Clinical risk factors and the radiomics signature were combined for multivariable logistic regression analysis, and radiomics nomogram model was then designed. The diagnostic performance of the model was evaluated using the receiver operating characteristic (ROC) curve. The area under the curve (AUC), sensitivity, and specificity were also calculated. We also assessed the performance of the radiomics nomogram with the decision curve analysis (DCA).Results
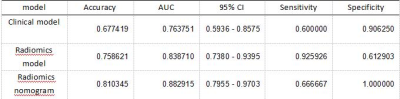
The model based on the radiomic features achieved an AUC of 0.84, accuracy, sensitivity and specificity of 76%, 93% and 61%, respectively in the test set, which show a better performance than that of the clinical model (AUC=0.73, 95% CI 0.59 - 0.86 in the test set) [Figure1, Table 1]. The combined radiomics nomogram model based on the radiomics and clinical features had the highest efficacy in detecting aMCI, with an AUC of 0.88, and the accuracy, sensitivity and specificity of 81%, 67%, and 100%, respectively in the test set [Figure1, Table 1]. The decision curve demonstrated that combined nomogram model had more favorable clinical predictive value than clinical model and radiomics model.Discussion
We have extracted quantitative radiomics features based on the 3D-T1WI imaging, including the texture analysis , histogram, shape-based features, texture-based features, wavelet features, Gray Level Co-Occurrence Matrix (GLCM), and Run-Length Matrix (RLM).These features can provide high quantitative image details in the frontal lobe. At the same time, previous studies have proved that the damage of the frontal-subcortical circuits and the destruction of white matter integrity, which have been associated with frontal executive function and processing speed compromises in aMCI and AD, are important reasons for the decline of cognitive function of aMCI and AD. The change of frontal lobe microenvironment of aMCI may be caused by those reasons. Additionally, the biological and epidemiological risk factors for disease from MCI to AD have been identified, including apolipoprotein E4 (APOE-e4), depression, loneliness, hearing impairment, diabetes, hypertension, older age, female gender and stronger cognitive impairment. Using univariate logistic regression, we found that age, hypertension and education level had high risk factor in our study. Because of using Chinese version of moka scale, education level is a significant factor. At last, the combined nomogram model showed excellent ability to distinguish the aMCI with the highest AUC of 0.88 in the testing set, which were higher than radiomics (testing set: AUC = 0.84) and clinical model (testing set: AUC = 0.73). Although the nomogram was not significantly better than radiomics, it still had higher values in accuracy (test set, nomogram vs radiomics, 81.03% vs.75.86%) and other diagnostic parameters, but it was obviously lower than radiomics model in sensitivity (test set, nomogram vs radiomics, 66.67% vs.92.59%).Conclusion
The combined radiomics nomogram model can be used to distinguish aMCI and CN with excellent diagnostic performance, which can be served as a potential decision support tool to assist clinicians in screening community aMCI population.Acknowledgements
This paper has not been presented anywhere, and is not being considered for publication elsewhere. There are no conflicts of interest for all authors with others. We have no relevant financial interests to disclose.References
[1] Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome [published correction appears in Arch Neurol 1999 Jun;56(6):760]. Arch Neurol. 1999;56(3):303-308. doi:10.1001/archneur.56.3.303.
[2] Zamani J, Sadr A, Javadi AH. Diagnosis of early mild cognitive impairment using a multiobjective optimization algorithm based on T1-MRI data. Sci Rep. 2022;12(1):1020. Published 2022 Jan 19. doi:10.1038/s41598-022-04943-3.
[3] Toshkhujaev S, Lee KH, Choi KY, et al. Classification of Alzheimer's Disease and Mild Cognitive Impairment Based on Cortical and Subcortical Features from MRI T1 Brain Images Utilizing Four Different Types of Datasets. J Healthc Eng. 2020;2020:3743171. Published 2020 Aug 31. doi:10.1155/2020/3743171.
[4] Turriziani P, Smirni D, Zappalà G, Mangano GR, Oliveri M, Cipolotti L. Enhancing memory performance with rTMS in healthy subjects and individuals with Mild Cognitive Impairment: the role of the right dorsolateral prefrontal cortex. Front Hum Neurosci. 2012;6:62. Published 2012 Apr 10. doi:10.3389/fnhum.2012.00062.
[5] Teipel S, Drzezga A, Grothe MJ, et al. Multimodal imaging in Alzheimer's disease: validity and usefulness for early detection. Lancet Neurol. 2015;14(10):1037-1053. doi:10.1016/S1474-4422(15)00093-9.
Figures

Figure 1. ROC analysis of different model (clinical model, radiomics model, radiomics nomogram based on the clinical and radiomics features) to distinguish aMCI from healthy volunteers in test set.